Pseudomonas aeruginosa Dnr-regulated denitrification in microoxic conditions
- PMID: 40772714
- PMCID: PMC12403760
- DOI: 10.1128/spectrum.00682-25
Pseudomonas aeruginosa Dnr-regulated denitrification in microoxic conditions
Abstract
Pseudomonas aeruginosa causes acute and chronic infections, such as those that occur in the lungs of people with cystic fibrosis (CF). In infection environments, oxygen (O2) concentrations are often low. The transcription factor Anr (
Keywords: Anr; Dnr; Pseudomonas aeruginosa; denitrification; microoxia; nitrate; nitric oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
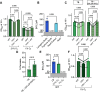

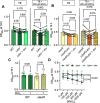
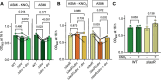

Update of
-
Role of Pseudomonas aeruginosa Dnr-regulated denitrification in oxic conditions.bioRxiv [Preprint]. 2025 Mar 31:2025.03.31.646406. doi: 10.1101/2025.03.31.646406. bioRxiv. 2025. Update in: Microbiol Spectr. 2025 Sep 2;13(9):e0068225. doi: 10.1128/spectrum.00682-25. PMID: 40236165 Free PMC article. Updated. Preprint.
References
-
- Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiss T, Botzenhart K, Yankaskas JR, Randell S, Boucher RC, Döring G. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest 109:317–325. doi: 10.1172/JCI0213870 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- P20 GM113132/GM/NIGMS NIH HHS/United States
- GREEN19G0/Cystic Fibrosis Foundation
- P20GM113132/GM/NIGMS NIH HHS/United States
- P30 CA023108/CA/NCI NIH HHS/United States
- P30 DK117469/DK/NIDDK NIH HHS/United States
- R21 AI174132/AI/NIAID NIH HHS/United States
- DK117469/DK/NIDDK NIH HHS/United States
- F31 AI179113/AI/NIAID NIH HHS/United States
- AI174132/National Institute of Allergy and Infectious Diseases
- BOMBER24P0/Cystic Fibrosis Foundation
- 5P30CA023108/CA/NCI NIH HHS/United States
- F31AI179113/National Institute of Allergy and Infectious Diseases
LinkOut - more resources
Full Text Sources

