Controversies in metastatic hormone-sensitive prostate cancer
- PMID: 40772795
- PMCID: PMC12330319
- DOI: 10.1002/cncr.70030
Controversies in metastatic hormone-sensitive prostate cancer
Abstract
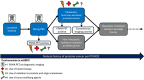
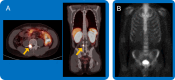
Metastatic hormone-sensitive prostate cancer (mHSPC) is an incurable phase of prostate cancer. Diagnostic tools and management strategies for this complex disease are expanding. Despite these advances, more therapies may not be the optimal approach for all patients. This review explores four major controversies surrounding mHSPC management-the role of prostate-specific membrane antigen positron emission tomography scans as diagnostic imaging, triplet therapy (androgen deprivation therapy, androgen receptor pathway inhibitor, and docetaxel chemotherapy), radiation to the prostate and/or oligo-metastases, and bone modifying agents. Critical evaluation of the data emphasizes the need for further work to determine which subgroups of patients with mHSPC benefit from each treatment. With a deeper understanding of these current issues, this review seeks to guide clinicians to refine their clinical practice to help patients achieve their best quantity and quality of life.
Keywords: PSMA PET; bone modifying agent; controversies; metastatic hormone‐sensitive prostate cancer; radiation; triplet therapy.
© 2025 The Author(s). Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Zachery Reichert reports advisory roles for AstraZeneca and Janssen; and research funding through the institution from AstraZeneca. The other authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

