Evolutionarily diverse caveolins share a common structural framework built around amphipathic disks
- PMID: 40772930
- PMCID: PMC12330381
- DOI: 10.1083/jcb.202411175
Evolutionarily diverse caveolins share a common structural framework built around amphipathic disks
Abstract
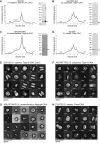
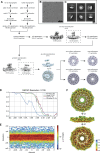
Caveolins are a unique family of membrane remodeling proteins present broadly across animals (Metazoa), and in vertebrates form flask-shaped invaginations known as caveolae. While human caveolin-1 assembles into an amphipathic disk composed of 11 spirally packed protomers, the structural basis underlying caveolin function across animals remains elusive. Here, we predicted structures for 73 caveolins spanning animal diversity, as well as a newly identified choanoflagellate caveolin from Salpingoeca rosetta. This analysis revealed seven conserved structural elements and a propensity to assemble into amphipathic disks. Cryo-EM structures of caveolins from S. rosetta choanoflagellate and the purple sea urchin Strongylocentrotus purpuratus exhibit striking structural similarities to human caveolin-1, validating the structural predictions. Lastly, tracing the chromosomal evolutionary history of caveolins revealed its parahoxozoan ancestral chromosome and evolutionary branches on which caveolins translocated and expanded. These results show that caveolins possess an ancient structural framework predating Metazoa and provide a new structural paradigm to explore the molecular basis of caveolin function across diverse evolutionary lineages.
© 2025 Han et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures
















References
MeSH terms
Substances
Grants and funding
- R01 GM080403/GM/NIGMS NIH HHS/United States
- R01HL144131/NH/NIH HHS/United States
- University of Virginia
- R01GM151635/NH/NIH HHS/United States
- University of Michigan Rackham Predoctoral Fellowship
- HHMI/Howard Hughes Medical Institute/United States
- R01 HL122010/HL/NHLBI NIH HHS/United States
- R01 HL144131/HL/NHLBI NIH HHS/United States
- T32GM007315/NH/NIH HHS/United States
- R01 GM129261/GM/NIGMS NIH HHS/United States
- R01HL122010/NH/NIH HHS/United States
- R01GM129261/NH/NIH HHS/United States
- R01GM080403/NH/NIH HHS/United States
- S10 OD030275/OD/NIH HHS/United States
- R01 GM151635/GM/NIGMS NIH HHS/United States
- 945026/European Research Council's Horizon 2020
- 905705/American Heart Association
- S10OD030275/NH/NIH HHS/United States
- Alexander von Humboldt Foundation
- T32 GM007315/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

