BLIMP-1 and CEACAM1 cooperatively regulate human Treg homeostasis and function to control xenogeneic GVHD
- PMID: 40773294
- PMCID: PMC12487853
- DOI: 10.1172/jci.insight.183676
BLIMP-1 and CEACAM1 cooperatively regulate human Treg homeostasis and function to control xenogeneic GVHD
Abstract
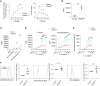
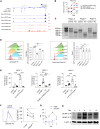
Regulatory T cells (Tregs) are essential for peripheral tolerance and depend on TCR and IL-2 receptor (IL-2R) signaling for their homeostasis and function. In mice, IL-2-dependent B-lymphocyte-induced maturation protein 1 (BLIMP-1) contributes to Treg homeostasis. BLIMP-1 is a major transcriptional hub in human Tregs, but its mechanisms of action remain undefined. Here, using CRISPR/Cas9 ablation, we show that BLIMP-1 limits human Treg proliferation but supports IL-10, cytotoxic T lymphocyte-associated protein 4, several immune checkpoints including carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), and Treg functional activity. BLIMP-1 restrains Treg expansion to IL-2 by downregulating CD25 and IL-2R signaling, and by enhancing CEACAM1 expression, which in turn inhibits responsiveness to CD3/CD28 signaling and activation of mTOR. Prolonged IL-2R signaling optimizes BLIMP-1 expression, supporting chromosomal opening of CEACAM1 to increased CEACAM1 expression through STAT5- and BLIMP-1-driven enhancers. Correspondingly, CEACAM1 is highly induced on Tregs from patients with autoimmune disease undergoing low-dose IL-2 therapy, and these Tregs showed reduced proliferation. A humanized mouse model of xenogeneic graft-versus-host disease demonstrates that BLIMP-1 normally promotes, while CEACAM1 restrains, Treg suppressive activity. Collectively, our findings reveal that BLIMP-1 and CEACAM1 function in an IL-2-dependent feedback loop to restrain Treg proliferation and affect suppressive function. CEACAM1 also acts as a highly selective biomarker of IL-2R signaling in human T cells.
Keywords: Adaptive immunity; Autoimmune diseases; Autoimmunity; Immunology; Immunotherapy.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

