A cholinergic spinal pathway for the adaptive control of breathing
- PMID: 40773345
- PMCID: PMC12466121
- DOI: 10.1016/j.celrep.2025.116078
A cholinergic spinal pathway for the adaptive control of breathing
Abstract
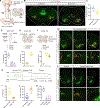
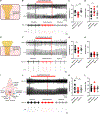
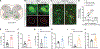
The ability to amplify motor neuron (MN) output is essential for generating high-intensity motor actions. This is critical for breathing that must be rapidly adjusted to accommodate changing metabolic demands. While brainstem circuits generate the breathing rhythm, the pathways that directly augment respiratory MN output are not well understood. Here, we map first-order inputs to phrenic motor neurons (PMNs), a key respiratory MN population that initiates diaphragm contraction to drive breathing. We identify a predominant spinal input from a distinct subset of genetically defined V0C cholinergic interneurons. We find that these interneurons receive phasic excitation from brainstem respiratory centers, augment phrenic output through M2 muscarinic receptors, and are highly activated under a hypercapnia challenge. Specifically silencing cholinergic interneuron neurotransmission impairs the breathing response to hypercapnia. Collectively, our findings identify a spinal pathway that amplifies breathing, presenting a potential target for promoting recovery of breathing following spinal cord injury.
Keywords: CP: Neuroscience; breathing; cholinergic interneurons; motor circuits; neuromodulation; phrenic motor neurons; spinal cord.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Update of
-
A cholinergic spinal pathway for the adaptive control of breathing.bioRxiv [Preprint]. 2025 Jan 20:2025.01.20.633641. doi: 10.1101/2025.01.20.633641. bioRxiv. 2025. Update in: Cell Rep. 2025 Aug 26;44(8):116078. doi: 10.1016/j.celrep.2025.116078. PMID: 39896653 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

