Triethylamine inhibits influenza A virus infection and growth via mechanisms independent of viral neuraminidase and RNA-dependent RNA polymerase
- PMID: 40773456
- PMCID: PMC12331046
- DOI: 10.1371/journal.pone.0329964
Triethylamine inhibits influenza A virus infection and growth via mechanisms independent of viral neuraminidase and RNA-dependent RNA polymerase
Abstract
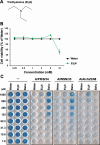
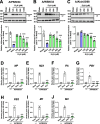
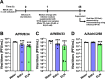
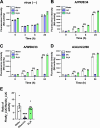
Triethylamine (Et3N) is a proton (H⁺) acceptor that is widely used in various industrial organic synthesis processes, including the production of pharmaceuticals, agrochemicals, and polymers. Inhalation of high Et3N concentrations can damage human respiratory tract and lungs. Given the compound's known reactivity and membrane-penetrating properties, we hypothesized that non-toxic concentrations of Et₃N may exert modulatory effects on virus-host interactions in epithelial cells. We thus investigated the anti-influenza activity of Et3N and found that it enhanced the viability of influenza A H1N1 and H3N2 virus-infected Madin-Darby canine kidney (MDCK) cells. Non-cytotoxic Et3N concentrations reduced the number of infected cells and suppressed influenza A virus nucleoprotein expression as well as viral gene and antiviral host gene upregulation in infected MDCK cells. Selectivity index values of Et₃N against influenza A virus infection, ranging from approximately 10 to over 50. These findings indicated that Et3N inhibited influenza A H1N1 and H3N2 viral infections. Additionally, Et3N suppressed influenza A H1N1 and H3N2 virus titers in the infected MDCK cell culture supernatant, suggesting that it inhibited viral growth in infected cells. This implies that Et3N may suppress influenza A virus release and/or replication by targeting viral or host cell factors. However, Et3N did not inhibit influenza A viral neuraminidase or RNA-dependent RNA polymerase activity, which are involved in viral release and replication, respectively. These results suggest that Et3N targets other viral proteins or host cell factors essential for influenza A virus growth. Our findings demonstrate that Et3N exerts anti-influenza activity, providing new insights into the development of antiviral agents based on Et3N skeleton.
Copyright: © 2025 Shoji et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Anti-influenza activity of Blumea Balsamifera (L.) DC. Extract: In vitro and in vivo evaluation against multiple influenza virus strains.Virus Res. 2025 Sep;359:199606. doi: 10.1016/j.virusres.2025.199606. Epub 2025 Jul 20. Virus Res. 2025. PMID: 40695408 Free PMC article.
-
In vitro antiviral activities of thymol and Limonin against influenza a viruses and SARS-CoV-2.Sci Rep. 2025 Jul 2;15(1):22587. doi: 10.1038/s41598-025-06967-x. Sci Rep. 2025. PMID: 40596436 Free PMC article.
-
Inhibitory effects of a hot-water extract of cumin fruit on influenza A virus infection.PLoS One. 2025 Jun 27;20(6):e0326423. doi: 10.1371/journal.pone.0326423. eCollection 2025. PLoS One. 2025. PMID: 40577311 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
-
Systematic review of influenza resistance to the neuraminidase inhibitors.BMC Infect Dis. 2011 May 19;11:134. doi: 10.1186/1471-2334-11-134. BMC Infect Dis. 2011. PMID: 21592407 Free PMC article.
References
-
- Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(1):1–24. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

