PPDPF promotes the progression of esophageal squamous cell carcinoma via c-Myc/CD24 axis
- PMID: 40774693
- PMCID: PMC12336582
- DOI: 10.1136/jitc-2025-012044
PPDPF promotes the progression of esophageal squamous cell carcinoma via c-Myc/CD24 axis
Abstract
Background: Despite a series of attempts during the last decades, the prognosis for esophageal squamous cell carcinoma (ESCC) remains poor. Although clinical immunotherapy trials have shown encouraging results, their benefits are limited. This study aims to identify novel targets for immunotherapy in ESCC.
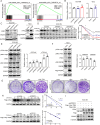
Experimental design: ESCC cell lines and mouse models were used to identify the tumor-promoting function of pancreatic progenitor cell differentiation and proliferation factor (PPDPF) and evaluate the effect of blockade of CD24. RNA sequencing was performed to profile transcriptomic changes upon PPDPF deficiency. Fluorescence microscopy-based phagocytosis assay and flow cytometry were employed to analyze macrophage phagocytosis. Immunoblotting, glutathione S-transferase-pulldown assay and co-immunoprecipitation assay were conducted to investigate the mechanism underlying the tumor-promoting role of PPDPF in ESCC. Clinical samples were analyzed to further validate the findings from preclinical models.
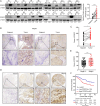
Results: The expression of PPDPF was significantly upregulated in ESCC. Deficiency of PPDPF inhibited the development of ESCC in mice. Mechanistically, PPDPF interfered with the c-Myc-GSK3β interaction and enhanced the protein stability of c-Myc, which increased the expression of CD24 and therefore promoted immune escape from macrophage phagocytosis. Positive correlations between PPDPF, c-Myc, and CD24 were observed in clinical samples. Anti-CD24 monotherapy effectively inhibited the ESCC tumor growth in mice.
Conclusions: PPDPF acts as an oncoprotein in ESCC by positively regulating the c-Myc/CD24 axis. These findings provide a potential effective target for immunotherapy in ESCC.
Keywords: Esophageal Cancer; Immune Checkpoint Inhibitor; Macrophage.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures





Similar articles
-
Hypoxia-induced PGK1 expression promotes esophageal squamous cell carcinoma progression via stimulating MYH9-mediated GSK3β/β-catenin signalling.Clin Transl Med. 2025 Jun;15(6):e70376. doi: 10.1002/ctm2.70376. Clin Transl Med. 2025. PMID: 40534132 Free PMC article.
-
WISP1 drives esophageal squamous cell carcinoma progression via modulation of cancer-associated fibroblasts and immune microenvironment.Front Immunol. 2025 Jul 23;16:1586790. doi: 10.3389/fimmu.2025.1586790. eCollection 2025. Front Immunol. 2025. PMID: 40771797 Free PMC article.
-
Targeting Drp1 inhibits ESCC progression via the ROS-PGC1-α-Nrf1/2 pathway.J Transl Med. 2025 Jun 17;23(1):674. doi: 10.1186/s12967-025-06697-8. J Transl Med. 2025. PMID: 40528181 Free PMC article.
-
YAP as a therapeutic target in esophageal squamous cell carcinoma: insights and strategies.Ann Med. 2025 Dec;57(1):2536200. doi: 10.1080/07853890.2025.2536200. Epub 2025 Jul 22. Ann Med. 2025. PMID: 40693409 Free PMC article. Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
