Cloning, Expression, and Characterization of GDSL-Type Lipolytic Enzyme Genes from Epidermidibacterium keratini EPI-7 Isolated from Human Skin
- PMID: 40774818
- PMCID: PMC12351110
- DOI: 10.4014/jmb.2504.04022
Cloning, Expression, and Characterization of GDSL-Type Lipolytic Enzyme Genes from Epidermidibacterium keratini EPI-7 Isolated from Human Skin
Abstract
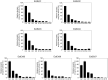
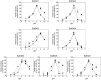
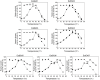
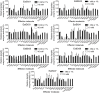
This study investigated seven putative lipolytic enzymes (EstEk01-07) from the skin microbiome bacterium Epidermidibacterium keratini EPI-7, focusing on their properties relevant to industrial applications. Sequence analysis revealed conserved GDSL motifs and four conserved blocks, characteristic of the GDSL/SGNH superfamily, with predicted α/β/α folds consistent with these enzymes. Significant variations in the number of α-helices and β-sheets among the EstEk enzymes suggested diverse substrate specificities and catalytic efficiencies. The enzymes exhibited a strong preference for short-chain fatty acids (C2-C4), classifying them as carboxylesterases, a novel finding within the skin microbiome. Optimal enzyme activity was observed at alkaline pH (8.0-9.0) and thermophilic condition (50-60°C), with substantial thermostability retained after heating at 50°C for three hours. Metal ion analysis revealed a significant stimulatory effect of Ca2+ and Fe3+, while other transition metals were inhibitory. The enzymes were stable in a range of non-ionic detergents, but sensitive to SDS. Moreover, they exhibited notable tolerance to various organic solvents, particularly methanol and isopropanol, suggesting potential applications in cosmetics and pharmaceutical industries. This study identifies a novel library of thermostable, alkaline carboxylesterases from the skin microbiome, highlighting their potential for industrial biocatalysis and further investigation into their role in skin lipid metabolism.
Keywords: Epidermidibacterium keratini; GDSL-type esterase/lipase; enzyme characterization; gene cloning; heterologous expression; skin microbiome.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures




Similar articles
-
Isolation, expression, and in silico profiling of a thermostable xylanase from Geobacillus stearothermophilus strain NASA267: insights into structural features and agro-waste valorization.Microb Cell Fact. 2025 Mar 21;24(1):69. doi: 10.1186/s12934-025-02672-6. Microb Cell Fact. 2025. Retraction in: Microb Cell Fact. 2025 Jul 10;24(1):161. doi: 10.1186/s12934-025-02790-1. PMID: 40119336 Free PMC article. Retracted.
-
Characterization of a Highly Solvent-Tolerant SGNH Hydrolase Superfamily Lipolytic Enzyme from Sphaerobacter thermophilus.Biochemistry. 2025 Jun 3;64(11):2412-2428. doi: 10.1021/acs.biochem.5c00057. Epub 2025 May 20. Biochemistry. 2025. PMID: 40392250
-
Data-Driven Mining of a Thermostable Lipase with Molecular Insights into Mechanisms of Its Substrate Specificity.J Agric Food Chem. 2025 Jul 16;73(28):17739-17749. doi: 10.1021/acs.jafc.5c02801. Epub 2025 Jul 1. J Agric Food Chem. 2025. PMID: 40590090
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Castilla A, Giordano SR, Irazoqui G. 2022. Chapter 16 - Extremophilic lipases and esterases: characteristics and industrial applications, pp. 207-222. In Kuddus M (ed.), Microbial Extremozymes, Ed. Academic Press. 10.1016/B978-0-12-822945-3.00001-4 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

