Protective Effect of N-Acetylcysteine (NAC) on oxLDL-Induced Endothelial Dysfunction
- PMID: 40774821
- PMCID: PMC12351112
- DOI: 10.4014/jmb.2504.04039
Protective Effect of N-Acetylcysteine (NAC) on oxLDL-Induced Endothelial Dysfunction
Abstract
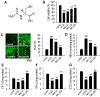
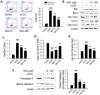
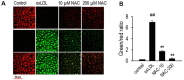
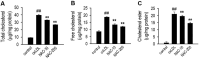
N-acetylcysteine (NAC), a well-known antioxidant and glutathione precursor, has been extensively studied for its free radical-scavenging properties, anti-inflammatory effects, and ability to enhance cellular redox balance. NAC has also been shown to mitigate oxidative damage in various disease models, yet its role in endothelial dysfunction remains underexplored. In this study, we evaluated the ability of NAC to counteract oxLDL-induced endothelial dysfunction in human umbilical vein endothelial cells (HUVECs). NAC treatment significantly reduced ROS levels, lipid peroxidation, and apoptotic markers while restoring mitochondrial membrane potential (MMP) and NO bioavailability. Additionally, NAC regulated the expression of eNOS, LOX-1, ICAM-1, and VCAM-1, demonstrating its role in reducing endothelial inflammation and improving vascular homeostasis. Furthermore, NAC prevented excessive cholesterol accumulation, suggesting its potential to regulate lipid metabolism in endothelial cells. These findings highlight the therapeutic potential of NAC in protecting against oxLDL-induced endothelial dysfunction and preventing vascular complications associated with cardiovascular diseases.
Keywords: Endothelial dysfunction; N-acetylcysteine; cardiovascular diseases; oxLDL; oxidative stress.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures

Similar articles
-
Glutathione as a Therapeutic Agent for OxLDL-Induced Endothelial Dysfunction and Atherosclerosis Prevention.Cell Biol Int. 2025 Sep;49(9):1163-1172. doi: 10.1002/cbin.70047. Epub 2025 Jun 13. Cell Biol Int. 2025. PMID: 40511641
-
Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells.J Vasc Surg. 2010 Nov;52(5):1290-300. doi: 10.1016/j.jvs.2010.04.085. Epub 2010 Aug 8. J Vasc Surg. 2010. PMID: 20692795
-
[Mechanism of salidroside in inhibiting expression of adhesion molecules in oxLDL-induced endothelial cells by regulating ferroptosis mediated by SIRT1/Nrf2].Zhongguo Zhong Yao Za Zhi. 2025 May;50(10):2787-2797. doi: 10.19540/j.cnki.cjcmm.20250312.301. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40686148 Chinese.
-
Antioxidant treatments for schizophrenia.Cochrane Database Syst Rev. 2016 Feb 5;2(2):CD008919. doi: 10.1002/14651858.CD008919.pub2. Cochrane Database Syst Rev. 2016. PMID: 26848926 Free PMC article.
-
A review of antioxidant N-acetylcysteine in addressing polycystic ovary syndrome.Gynecol Endocrinol. 2024 Dec;40(1):2381498. doi: 10.1080/09513590.2024.2381498. Epub 2024 Jul 22. Gynecol Endocrinol. 2024. PMID: 39039898
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

