Artificial transneurons emulate neuronal activity in different areas of brain cortex
- PMID: 40774953
- PMCID: PMC12332047
- DOI: 10.1038/s41467-025-62151-9
Artificial transneurons emulate neuronal activity in different areas of brain cortex
Abstract
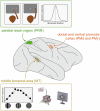
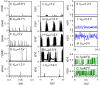
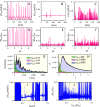
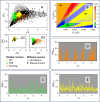
Rapid development of memristive elements emulating biological neurons creates new opportunities for brain-like computation at low energy consumption. A first step toward mimicking complex neural computations is the analysis of single neurons and their characteristics. Here we measure and model spiking activity in artificial neurons built using diffusive memristors. We compare activity of these artificial neurons with the spiking activity of biological neurons measured in sensory, pre-motor, and motor cortical areas of the monkey (male) brain. We find that artificial neurons can operate in diverse self-sustained and noise-induced spiking regimes that correspond to the activity of different types of cortical neurons with distinct functions. We demonstrate that artificial neurons can function as trans-functional devices (transneurons) that reconfigure their behaviour to attain instantaneous computational needs, each capable of emulating several biological neurons.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Inclusion & Ethics: The authors have carefully considered researcher contributions and authorship criteria of multi-region collaboration to promote greater equity in this collaborative project. Experimental protocols for measurement of brain activity in macaque monkeys were approved by the Animal Care and Use Committee of the Salk Institute (MT data) and Institutional Animal Care and Use Committee of the Washington University (PRR data); these protocols conform to U.S. Department of Agriculture regulations and to the National Institutes of Health guidelines for the humane care and use of laboratory animals.
Figures


References
-
- Yu, Q., Tang, H., Hu, J. & Chen, K. T. Neuromorphic Cognitive Systems (Springer, 2017).
-
- Aimone, J. B. A roadmap for reaching the potential of brain-derived computing. Adv. Intell. Syst.3, 2000191 (2021).
-
- Ham, D., Park, H., Hwang, S. & Kim, K. Neuromorphic electronics based on copying and pasting the brain. Nat. Electron.4, 635 (2021).
-
- Barlow, H. B. Single units and sensation: a neuron doctrine for perceptual psychology? Perception1, 371–394 (1972). - PubMed
-
- Hubel, D. H & Wiesel, T. N. Brain and Visual perception: The story of a 25-year collaboration (Oxford University Press, 2005). .
MeSH terms
Grants and funding
- EP/S032843/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
- R01 EY018613; P30 EY019005/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01-EY029117/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY018613/EY/NEI NIH HHS/United States
- R01 NS123435/NS/NINDS NIH HHS/United States
- R01 EY029117/EY/NEI NIH HHS/United States
- P30 EY019005/EY/NEI NIH HHS/United States
- R01 EB028154/EB/NIBIB NIH HHS/United States
- FA9550-19-1-0213/United States Department of Defense | United States Air Force | AFMC | Air Force Office of Scientific Research (AF Office of Scientific Research)
- R01 EY012135/EY/NEI NIH HHS/United States
- 1R01EB028154/U.S. Department of Health & Human Services | NIH | National Institute of Biomedical Imaging and Bioengineering (NIBIB)
LinkOut - more resources
Full Text Sources

