A pharmacovigilance study of vortioxetine based on data from the FDA adverse event reporting system
- PMID: 40775011
- PMCID: PMC12332032
- DOI: 10.1038/s41598-025-13786-7
A pharmacovigilance study of vortioxetine based on data from the FDA adverse event reporting system
Abstract
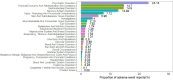
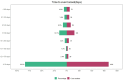
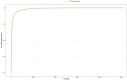
Vortioxetine is an antidepressant approved for the treatment of major depressive disorder (MDD). Given its widespread post-marketing clinical use, it is essential to explore its real-world safety. Reports were extracted from the FDA Adverse Event Reporting System (FAERS) from the third quarter of 2013 to the first quarter of 2025. Four disproportionality analysis methods, commonly used in pharmacovigilance to evaluate the relative reporting frequency of adverse events (AEs), were employed to identify AE signals associated with vortioxetine. These included the Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Multi-item Gamma Poisson Shrink (MGPS), and Bayesian Confidence Propagation Neural Network (BCPNN). The median was used to describe the time to onset (TTO) of AEs, and Weibull distribution was employed to assess the trend of AE occurrence over time. In addition, sensitivity analyses were conducted to ensure the robustness of the findings. A total of 13,613 individual case safety reports (ICSRs) involving 34,156 AEs were analyzed. Females accounted for 60.9% of the reports, while males represented 26.5%. The median age of patients was 42 years (interquartile range: 26-59 years), with most cases (34.1%) in the 18-65 age group. The United States contributed the highest proportion of reports (77.4%). Common AEs included nausea (n = 2042, ROR = 5.11, PRR = 4.86, EBGM = 4.85, IC = 2.28), anxiety (n = 781, ROR = 5.3, PRR = 5.2, EBGM = 5.18, IC = 2.37 ), vomiting (n = 773, ROR = 3.23, PRR = 3.17, EBGM = 3.17, IC = 1.66), headache (n = 670, ROR = 1.96, PRR = 1.94, EBGM = 1.94, IC = 0.96), and somnolence (n = 212, ROR = 2, PRR = 1.99, EBGM = 1.99, IC = 0.99). Notably, several AEs not listed on the drug label, such as tinnitus (n = 79, ROR = 3.24, PRR = 3.24, EBGM = 3.23, IC = 1.69), urinary retention (n = 62, ROR = 3.57, PRR = 3.57, EBGM = 3.56, IC = 1.83), prolonged QT interval (n = 62, ROR = 3.14, PRR = 3.13, EBGM = 3.13, IC = 1.64), and restless legs syndrome (n = 48, ROR = 5.08, PRR = 5.08, EBGM = 5.06, IC = 2.34) were also identified. Most AEs occurred within the first month of treatment, with a median onset time of 15 days. Sensitivity analyses confirmed the consistency of these findings. This study provides new insights into the safety of vortioxetine and offers preliminary safety evidence. In addition, the findings may inform updates to prescribing information and guide post-marketing safety surveillance. However, the spontaneous nature of the FAERS database precludes establishing a causal relationship between vortioxetine and the reported AEs. Further prospective studies are needed to validate our findings.
Keywords: Adverse events; Disproportionality analysis; FAERS; Pharmacovigilance; Vortioxetine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Adverse drug reactions related to methotrexate: a real-world pharmacovigilance study using the FAERS database from 2004 to 2024.Front Immunol. 2025 Jun 4;16:1586361. doi: 10.3389/fimmu.2025.1586361. eCollection 2025. Front Immunol. 2025. PMID: 40534848 Free PMC article. Review.
-
Unveiling potential adverse events associated with escitalopram oxalate: A real-world analysis based FDA adverse event reporting system database.J Psychopharmacol. 2024 Jun;38(6):567-578. doi: 10.1177/02698811241249651. Epub 2024 Apr 27. J Psychopharmacol. 2024. PMID: 38678377
-
Adverse events associated with gepants: a pharmacovigilance analysis based on the FDA adverse event reporting system.J Headache Pain. 2025 Jun 23;26(1):147. doi: 10.1186/s10194-025-02091-3. J Headache Pain. 2025. PMID: 40551098 Free PMC article.
-
Safety evaluation of irinotecan: a real-world disproportionality analysis using FAERS and JADER databases during the time period 2004-2024.Front Pharmacol. 2025 Jun 9;16:1516449. doi: 10.3389/fphar.2025.1516449. eCollection 2025. Front Pharmacol. 2025. PMID: 40552159 Free PMC article.
-
Comparative Analysis of Adverse Events Linked to PEG-rhG-CSF and rhG-CSF in Real-World Settings: Disproportionate Examination of the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) Database.Clin Ther. 2025 Aug;47(8):624-630. doi: 10.1016/j.clinthera.2025.04.017. Epub 2025 May 22. Clin Ther. 2025. PMID: 40410065 Review.
References
-
- Theunissen, E. L. et al. A randomized trial on the acute and steady-state effects of a new antidepressant, Vortioxetine (Lu AA21004), on actual driving and cognition. Clin. Pharmacol. Ther.93, 493–501 (2013). - PubMed
-
- Christensen, M. C. et al. Clinical benefits of Vortioxetine 20 mg/day in patients with major depressive disorder. CNS Spectr.28, 693–701 (2023). - PubMed
-
- Verrienti, G., Colonna, I. & Raccagni, C. Use of Vortioxetine in different neurological fields: a systematic review and future perspectives. Neurol. Sci.10.1007/s10072-025-07987-1 (2025). - PubMed
-
- McIntyre, R. S., Lee, Y. & Mansur, R. B. Treating to target in major depressive disorder: response to remission to functional recovery. CNS Spectr.20, 17–31 (2015). - PubMed
-
- Xue, L., Lewis, E., Bocharova, M., Young, A. H. & Aarsland, D. Decreased neutrophil-to-lymphocyte ratio predicted cognitive improvement in late-life depression treated with vortioxetine: findings from an eight-week randomized controlled trial. Brain Behav. Immun.126, 53–58 (2025). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

