The efficacy and safety of inhaled peptide YKYY017 for COVID-19 patients with mild illness: a phase 2 randomized controlled trial
- PMID: 40775020
- PMCID: PMC12332093
- DOI: 10.1038/s41467-025-62214-x
The efficacy and safety of inhaled peptide YKYY017 for COVID-19 patients with mild illness: a phase 2 randomized controlled trial
Abstract
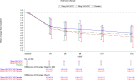
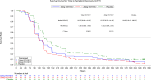
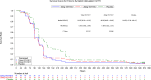
YKYY017 is a SARS-CoV-2 membrane fusion inhibitor. We report efficacy and safety of inhaled YKYY017 for COVID-19 patients with mild to moderate illness from a phase 2 trial (ChiCTR2300075467). 239 patients aged 18-75 years with mostly mild COVID-19 were randomly allocated to receive aerosol inhalation of 10 or 20 mg YKYY017 or placebo once daily. The primary endpoint is the change in SARS-CoV-2 viral load from baseline to Day 4. The mean (±SE) differences in viral load change from baseline were -0.48 ± 0.27 log10 copies/mL (95% CI, -1.01 to 0.06) for the 20 mg group and -0.27 ± 0.27 log10 copies/mL (95% CI, -0.79 to 0.26) for the 10 mg group, compared to the placebo group. Viral load changes at visits other than Day 4 did not differ significantly from placebo in either the 10 or 20 mg YKYY017 groups. The time to sustained symptom recovery was shorter in the 20 mg YKYY017 group (median 117.53, 95%CI 95.33 to 141.45 hours) than in the placebo group (median 143.00, 95%CI 139.17 to 186.87 hours; HR 1.552, 95%CI 1.089 to 2.214, p = 0.0151), whereas the 10 mg YKYY017 group showed a similar but not statistically significant trend compared to placebo (p = 0.0833). The time to sustained symptom alleviation was shorter in both the 20 and 10 mg YKYY017 groups than in the placebo group. The adverse events were mostly mild to moderate. The primary outcome was not met. Following a supplementary phase 1b trial, we are planning another phase 2/3 trial using a twice-daily 20 mg YKYY017 regimen to further assess efficacy and safety.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Bin Cao discloses his role as PI in China for the baloxavir transmission trial sponsored by Roche and as PI of GP681 Phase II and Phase III trials sponsored by Qingfeng Pharmaceutical Group Co., Ltd. Yeming Wang serves as the sub-PI. Yeming Wang also discloses receiving compensation as rapporteur for “Optimizing the Clinical Management of Patients” in the 2024 update of the WHO Public Health Research Agenda for Influenza, led by WHO. Gengshen Song and Xia Wang are employees of Youcare Pharmaceutical Co., Ltd. Jie Zhai is an employee of Beijing Yolax Pharmaceutical Technology Co., Ltd. The remaining authors declare no competing interests.
Figures

Similar articles
-
Nirmatrelvir-ritonavir versus placebo-ritonavir in individuals with long COVID in the USA (PAX LC): a double-blind, randomised, placebo-controlled, phase 2, decentralised trial.Lancet Infect Dis. 2025 Aug;25(8):936-946. doi: 10.1016/S1473-3099(25)00073-8. Epub 2025 Apr 3. Lancet Infect Dis. 2025. PMID: 40188838 Clinical Trial.
-
Efficacy and safety of SHEN26, a novel oral small molecular RdRp inhibitor for COVID-19 treatment: a multicenter, randomized, double-blinded, placebo-controlled, phase II clinical trial.Virol J. 2025 Jan 25;22(1):16. doi: 10.1186/s12985-025-02631-y. Virol J. 2025. PMID: 39863888 Free PMC article. Clinical Trial.
-
Efficacy and safety of onradivir in adults with acute uncomplicated influenza A infection in China: a multicentre, double-blind, randomised, placebo-controlled and oseltamivir-controlled, phase 3 trial.Lancet Respir Med. 2025 Jul;13(7):597-610. doi: 10.1016/S2213-2600(25)00046-3. Epub 2025 Jun 7. Lancet Respir Med. 2025. PMID: 40489986 Clinical Trial.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
-
- WHO. Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic, https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meet...-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (2023).
-
- Davido, B., Megarbane, B. & Loubet, P. COVID-19 surge during summer 2024: the phantom menace? Clin Microbiol Infect10.1016/j.cmi.2024.07.009 (2024). - PubMed
-
- Zheng, B. et al. Small-molecule antiviral treatments for COVID-19: A systematic review and network meta-analysis. Int J. Antimicrob. Agents63, 107096 (2024). - PubMed
-
- Andrews, H. S., Herman, J. D. & Gandhi, R. T. Treatments for COVID-19. Annu Rev. Med.75, 145–157 (2024). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

