Metabolic dynamics of human external urethral sphincter myoblast differentiation and the effects of tricarboxylic acid cycle inhibition
- PMID: 40775021
- PMCID: PMC12331984
- DOI: 10.1038/s41598-025-13764-z
Metabolic dynamics of human external urethral sphincter myoblast differentiation and the effects of tricarboxylic acid cycle inhibition
Abstract
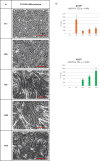
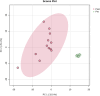
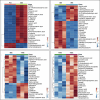
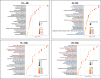
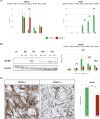
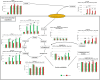
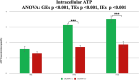
Stress urinary incontinence commonly arises with aging or following prostatectomy, yet its underlying mechanisms remain unclear. To address this, we investigated the role of metabolic pathways-particularly the tricarboxylic acid (TCA) cycle-in the differentiation of human external urethral sphincter myoblasts. Immortalized sphincter cells (US2-KD) were induced to differentiate over 192 h. Metabolomic profiling using gas chromatography-mass spectrometry, along with pathway enrichment analysis, identified key metabolic changes. Inhibition of mitochondrial pyruvate transport with UK5099 markedly suppressed TCA cycle metabolites, including citrate, α-ketoglutarate, fumarate, and malate. This inhibition also significantly reduced MYH7 expression and intracellular adenosine triphosphate levels throughout the differentiation period. These results demonstrate that the TCA cycle plays a critical role in both energy metabolism and the differentiation of urethral sphincter myoblasts. This study is the first to suggest that impaired TCA cycle activity may contribute to the pathogenesis of Stress urinary incontinence and represents a potential therapeutic target. Our findings offer new insight into age-related metabolic decline associated with Stress urinary incontinence and support the development of therapies that combine metabolic modulation with regenerative approaches.
Keywords: External urethral sphincter; Metabolomic pathways; Myoblast differentiation; Stress urinary incontinence; Tricarboxylic acid cycle.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Retropubic or transobturator mid-urethral slings for intrinsic sphincter deficiency-related stress urinary incontinence in women: a systematic review and meta-analysis.Int Urogynecol J. 2016 Jan;27(1):19-28. doi: 10.1007/s00192-015-2797-3. Epub 2015 Jul 29. Int Urogynecol J. 2016. PMID: 26220506
-
Umbelliferone attenuates diabetic sarcopenia by modulating mitochondrial quality and the ubiquitin-proteasome system.Phytomedicine. 2025 Aug;144:156930. doi: 10.1016/j.phymed.2025.156930. Epub 2025 May 31. Phytomedicine. 2025. PMID: 40483791
-
Single-incision sling operations for urinary incontinence in women.Cochrane Database Syst Rev. 2017 Jul 26;7(7):CD008709. doi: 10.1002/14651858.CD008709.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Oct 27;10:CD008709. doi: 10.1002/14651858.CD008709.pub4. PMID: 28746980 Free PMC article. Updated.
-
Use of endoanal ultrasound for reducing the risk of complications related to anal sphincter injury after vaginal birth.Cochrane Database Syst Rev. 2015 Oct 29;2015(10):CD010826. doi: 10.1002/14651858.CD010826.pub2. Cochrane Database Syst Rev. 2015. PMID: 26513224 Free PMC article.
-
Single-incision sling operations for urinary incontinence in women.Cochrane Database Syst Rev. 2014 Jun 1;(6):CD008709. doi: 10.1002/14651858.CD008709.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Jul 26;7:CD008709. doi: 10.1002/14651858.CD008709.pub3. PMID: 24880654 Updated.
References
-
- Patel, U. J., Godecker, A. L., Giles, D. L. & Brown, H. W. Updated prevalence of urinary incontinence in women: 2015–2018 National population-based survey data. Female Pelvic Med. Reconstr. Surg.28 (4), 181–187 (2022). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

