Galectin-3-integrin α5β1 phase separation disrupted by advanced glycation end-products impairs diabetic wound healing in rodents
- PMID: 40775187
- PMCID: PMC12332078
- DOI: 10.1038/s41467-025-62320-w
Galectin-3-integrin α5β1 phase separation disrupted by advanced glycation end-products impairs diabetic wound healing in rodents
Abstract
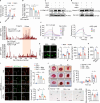
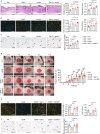
Diabetic foot ulcers are severe diabetic complications, and promoting impaired angiogenesis is essential for wound healing. Pro-angiogenic galectin-3 is elevated in diabetic serum and promotes systemic insulin resistance that may impair wound healing. However, the exact role of galectin-3 in the regulation of diabetic wound healing remains unclear. Here, we demonstrate that galectin-3 promotes skin wound healing and angiogenesis via binding to its receptor integrin α5β1, and enhances downstream focal adhesion kinase phosphorylation by forming a liquid-liquid phase separation with integrin α5β1. Under diabetic conditions, aberrant accumulated advanced glycation end-products bind to galectin-3, blocking its interaction with integrin α5β1 and impairing angiogenesis. Topical treatment of recombinant galectin-3 in hydrogels promotes diabetic wound healing in rodents without causing systemic insulin resistance and synergizes with insulin. This study clarifies the binding of galectin-3 to integrin α5β1, instead of advanced glycation end-products, forming phase separation to promote angiogenesis and diabetic wound healing, laying the foundation for local galectin-3 therapy to treat diabetic foot ulcers.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Walsh, J. W., Hoffstad, O. J., Sullivan, M. O. & Margolis, D. J. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet. Med33, 1493–1498 (2016). - PubMed
-
- Dixon, D. & Edmonds, M. Managing diabetic foot ulcers: pharmacotherapy for wound Healing. Drugs81, 29–56 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

