Opportunities for and Challenges of Pulmonary Drug Delivery in the Management of Acute Exacerbations of CNS Disorders
- PMID: 40775197
- PMCID: PMC12515229
- DOI: 10.1007/s40263-025-01213-4
Opportunities for and Challenges of Pulmonary Drug Delivery in the Management of Acute Exacerbations of CNS Disorders
Erratum in
-
Correction: Opportunities for and Challenges of Pulmonary Drug Delivery in the Management of Acute Exacerbations of CNS Disorders.CNS Drugs. 2025 Nov;39(11):1193-1194. doi: 10.1007/s40263-025-01224-1. CNS Drugs. 2025. PMID: 40944821 Free PMC article. No abstract available.
Abstract
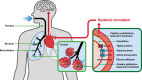
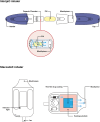
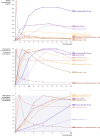
Advances in pulmonary (PM) drug delivery through inhalation devices have enabled effective treatments for acute exacerbations of central nervous system (CNS) episodes, addressing previously unmet medical needs. While PM formulations of loxapine and levodopa are approved for agitation and off periods in Parkinson's disease (PD), respectively, there remains an unmet need for rapid-acting therapies for other acute exacerbations of neurologic disorders. In this review, the potential of PM delivery to address this gap in the management of acute CNS disorders is critically assessed, focusing on Staccato® loxapine for agitation, Inbrija® (levodopa) for PD, the investigational drug inhaler device Staccato® alprazolam for epilepsy, and other investigational drug inhaler devices. PM delivery benefits from bypassing first-pass metabolism, utilizing inhalation devices to enable rapid drug delivery to the densely perfused alveolar space, arterial bloodstream, and brain. However, challenges include lung tissue sensitivity, low dose volume (compared with oral and intravenous administration), and difficulties with administration during certain acute episodes. Pharmacokinetic, efficacy, and safety data from approved or investigational PM therapies for agitation, PD, epilepsy, migraine, and insomnia present inhalation as a promising option for patients requiring acute episode management by facilitating fast absorption and onset of action and generally good tolerability. In particular, for epilepsy, on-demand medication that may be administered by patients or caregivers early at seizure onset may translate to improved patient outcomes. To enhance PM management of acute exacerbations of CNS disorders, further research and user training for optimal PM administration are required.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: The authors received no funding for the development of this work. Medical writing and creative and editorial assistance were provided by Ogilvy Health UK and funded by UCB. The open access fee was paid by UCB. Conflicts of interest: K.D. has received honorarium from Crossject, Neurelis, and UCB for participating in advisory board meetings. AS has received personal fees and grants from Angelini Pharma, Biocodex, Desitin Arzneimittel, Eisai, Jazz Pharmaceuticals, Neuraxpharm, Takeda, UCB Pharma, and UNEEG Medical. J.C.C. serves as an advisory board member for Neurelis, serves as a consultant for Crossject, and has received research grants from PrevEp and Allaysis. R.R., C.L., and H.C. are employed by UCB. Ethics approval: Not applicable. Consent to participate: Not applicable. Consent for publication: Not applicable. Availability of data and material: Not applicable. Code availability: Not applicable. Author contributions: K.D.: conceptualization, writing—review and editing. A.S.: conceptualization, writing—review and editing. R.R.: conceptualization, writing—review and editing. C.L.: conceptualization, writing—review and editing, funding acquisition. H.C.: conceptualization, writing—review and editing. J.C.C.: conceptualization, writing—review and editing. All authors have read and approved the final submitted manuscript and agree to be accountable for the work.
Figures
References
-
- Penovich PE, Buelow J, Steinberg K, Sirven J, Wheless J. Burden of seizure clusters on patients with epilepsy and caregivers: survey of patient, caregiver, and clinician perspectives. Neurologist. 2017;22(6):207–14. 10.1097/nrl.0000000000000140. - PubMed
-
- Sachs GS. A review of agitation in mental illness: burden of illness and underlying pathology. J Clin Psychiatry. 2006;67(Suppl 10):5–12. - PubMed
-
- Tanner CM. Exploring the clinical burden of OFF periods in Parkinson disease. Am J Manag Care. 2020;26(12 Suppl):S255–64. 10.37765/ajmc.2020.88517. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

