Efficacy of modified-vaccinia Ankara vaccine as pre- and post-exposure prophylaxis against monkeypox sexual transmission in non-human primate model
- PMID: 40775237
- PMCID: PMC12332035
- DOI: 10.1038/s41467-025-62681-2
Efficacy of modified-vaccinia Ankara vaccine as pre- and post-exposure prophylaxis against monkeypox sexual transmission in non-human primate model
Abstract
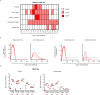
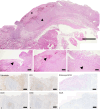
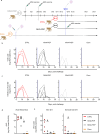
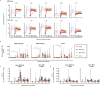
The monkeypox virus (MPXV) outbreak of 2022 caused a human disease with unusual epidemiological and clinical features, notably an increase in human-to-human transmission through sexual contact, predominantly among men who have sex with men (MSM). This evolution underscores the need to reassess prevention and control strategies in the context of a sexually transmitted disease. Here, we show that rectal challenge of male cynomolgus macaques with a 2022 clade IIb MPXV isolate mimics sexual transmission, leading to rectal infection, with systemic and male genital tract dissemination and seminal fluid shedding. Vaccination with modified-vaccinia Ankara (MVA) protected the macaques from subsequent rectal MPXV challenge. However, MVA failed to prevent the disease when administered four days post-exposure to MPXV. These findings have a critical impact on outbreak management and highlight the importance of reevaluating MVA post-exposure prophylaxis protocols.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Nathalie Silvestre and Catherine Brua are Transgene employees and may hold shares and/or stock options in the company. The remaining authors declare no competing interests.
Figures

Similar articles
-
Correlation between microneutralization test and a multiplexed immunoassay for evaluation of monkeypox and vaccinia virus antibodies before and after smallpox vaccination.Front Immunol. 2025 Jun 23;16:1585284. doi: 10.3389/fimmu.2025.1585284. eCollection 2025. Front Immunol. 2025. PMID: 40625755 Free PMC article.
-
Characterization and comparison of immunity against MPXV for individuals infected with MPXV or vaccinated with modified vaccinia Ankara vaccines.J Immunol. 2025 Feb 1;214(2):211-222. doi: 10.1093/jimmun/vkae031. J Immunol. 2025. PMID: 40073241
-
Mpox Clinical Presentation, Diagnostic Approaches, and Treatment Strategies: A Review.JAMA. 2024 Nov 19;332(19):1652-1662. doi: 10.1001/jama.2024.21091. JAMA. 2024. PMID: 39401235 Review.
-
Tiantan vaccinia virus-based vaccine with promising safety provides sustained protection against mpox in non-human primates.Nat Commun. 2025 Aug 5;16(1):7183. doi: 10.1038/s41467-025-62594-0. Nat Commun. 2025. PMID: 40764511 Free PMC article.
-
Exploring monkeypox virus antibody levels: insights from human immunological research.Virol J. 2025 May 31;22(1):175. doi: 10.1186/s12985-025-02748-0. Virol J. 2025. PMID: 40450351 Free PMC article. Review.
References
-
- Gessain, A., Nakoune, E. & Yazdanpanah, Y. Monkeypox. N. Engl. J. Med.387, 1783–1793 (2022). - PubMed
-
- World-Health-Organization. WHO Director-General declares the ongoing monkeypox outbreak a Public Health Emergency of International Concern. https://www.who.int/azerbaijan/news/item/23-07-2022-who-director-general..., (2022).
-
- Thornhill, J. P., Antinori, A. & Orkin, C. M. Monkeypox virus infection across 16 countries - April-June 2022. Reply. N. Engl. J. Med.387, e69 (2022). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

