Structural Insights into the Protein Mannosyltransferase from Mycobacterium tuberculosis reveal a WW-Domain-Like Protein Motif in Bacteria
- PMID: 40775265
- PMCID: PMC12331936
- DOI: 10.1038/s42003-025-08593-9
Structural Insights into the Protein Mannosyltransferase from Mycobacterium tuberculosis reveal a WW-Domain-Like Protein Motif in Bacteria
Abstract
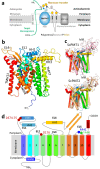
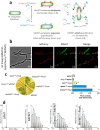
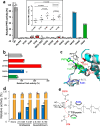
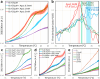
We have previously demonstrated that protein-O-mannosylation (POM), a widespread post-translational glycosyl modification of proteins, is a key virulence factor of Mycobacterium tuberculosis (Mtb), the world's deadliest infectious agent. Here, we report a detailed analysis of the structure-function relationship of MtPMT, the enzyme that catalyzes POM in Mtb. Using mutagenesis and in cellulo monitoring of POM activity, we demonstrate that, despite notable structural differences, MtPMT shares functional homologies with yeasts' PMTs in the mechanism of the sugar transfer from lipidic donors. Furthermore, we provide evidence that the selectivity for proline-rich target glycosylation sites that differentiates MtPMT from its eukaryotic homologues, relies on a WW-like domain, which preferentially interacts with proline-rich acceptor substrate analogues. This first identification of a functional WW-like domain in a prokaryotic protein raises questions about its potential evolutionary linkage with eukaryotic WW modules and provides new insights into PMT's acceptor-substrate recognition mechanism paving the way for the development selective inhibitors of MtPMT with potential therapeutic application against tuberculosis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: I declare the authors have no competing interests as defined by Nature Research or other interests that might be perceived to influence the interpretation of the article.
Figures



Similar articles
-
Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance.Cochrane Database Syst Rev. 2018 Aug 27;8(8):CD012768. doi: 10.1002/14651858.CD012768.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2021 Jan 15;1:CD012768. doi: 10.1002/14651858.CD012768.pub3. PMID: 30148542 Free PMC article. Updated.
-
Diverse impacts of different rpoB mutations on the anti-tuberculosis efficacy of capreomycin.EBioMedicine. 2025 Jul;117:105776. doi: 10.1016/j.ebiom.2025.105776. Epub 2025 May 30. EBioMedicine. 2025. PMID: 40449326 Free PMC article.
-
Ser/Thr phosphorylation of Mycobacterium tuberculosis type II RelK toxin by PknK destabilizes TA interaction and interferes with toxin neutralization.mBio. 2025 Jul 9;16(7):e0106825. doi: 10.1128/mbio.01068-25. Epub 2025 Jun 17. mBio. 2025. PMID: 40525867 Free PMC article.
-
Mechanistic Insights into Homoserine O-Acetyltransferase from Mycobacterium tuberculosis.Biochemistry. 2025 Aug 5;64(15):3299-3310. doi: 10.1021/acs.biochem.5c00089. Epub 2025 Jul 22. Biochemistry. 2025. PMID: 40695725
-
Xpert MTB/XDR for detection of pulmonary tuberculosis and resistance to isoniazid, fluoroquinolones, ethionamide, and amikacin.Cochrane Database Syst Rev. 2022 May 18;5(5):CD014841. doi: 10.1002/14651858.CD014841.pub2. Cochrane Database Syst Rev. 2022. PMID: 35583175 Free PMC article.
References
-
- Hang, J. et al. Protein O-mannosylation across kingdoms and related diseases: from glycobiology to glycopathology. Biomed. Pharmacother.148, 112685 (2022). - PubMed
-
- Cowlishaw, D. A. & Smith, M. C. Glycosylation of a Streptomyces coelicolor A3(2) cell envelope protein is required for infection by bacteriophage phi C31. Mol. Microbiol.41, 601–610 (2001). - PubMed
-
- Mahne, M., Tauch, A., Puhler, A. & Kalinowski, J. The Corynebacterium glutamicum gene pmt encoding a glycosyltransferase related to eukaryotic protein-O-mannosyltransferases is essential for glycosylation of the resuscitation promoting factor (Rpf2) and other secreted proteins. FEMS Microbiol. Lett.259, 226–233 (2006). - PubMed
-
- VanderVen, B. C., Harder, J. D., Crick, D. C. & Belisle, J. T. Export-mediated assembly of mycobacterial glycoproteins parallels eukaryotic pathways. Science309, 941–943 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

