Get strong to fight childhood cancer - an exercise intervention for children and adolescents undergoing anti-cancer treatment (FORTEe): Rationale and design of a randomized controlled exercise trial
- PMID: 40775303
- PMCID: PMC12330123
- DOI: 10.1186/s12885-025-14489-y
Get strong to fight childhood cancer - an exercise intervention for children and adolescents undergoing anti-cancer treatment (FORTEe): Rationale and design of a randomized controlled exercise trial
Abstract
Background: Despite substantial advances in treatment, children and adolescents with cancer continue to face high morbidity and health issues, including cancer-related fatigue, treatment-related complications, and physical inactivity. Integrating exercise into pediatric oncology care has emerged as a promising approach to mitigate these burdens during cancer treatment. While preliminary data support its potential to reduce treatment-related side effects and enhance quality of life, robust evidence -especially from large, multicenter trials- remains limited.
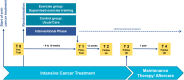
Methods: The FORTEe trial is a randomized, controlled, multicenter trial evaluating a personalized and standardized exercise intervention powered to include 450 children, adolescents, and young adults undergoing cancer treatment across ten centers in Europe. The trial aims to provide high-quality evidence for integrating precision exercise therapy as part of standard care. Participants are randomly assigned to either the exercise intervention group, receiving a tailored, supervised 8-10 weeks lasting exercise program, or the control group, receiving usual care. The exercise program includes endurance, strength, flexibility, and balance training, adapted to each patient's age, fitness, and cancer treatment phase. Exercise sessions are intended to take place 3-5 times a week with moderate intensity, with both frequency and intensity adapted to the clinical condition of the individual. Digital tools and telehealth solutions support the intervention, allowing for both in-person and remote training.
Discussion: With a target enrolment of 450 patients, the FORTEe trial will be one of the largest interventional studies in pediatric exercise oncology. Given that childhood cancer is a rare disease, this sample size is only achievable through a multicenter approach. Enhancing statistical power, the large sample will enable more robust analyses of the intervention's effects in a diverse population across multiple European centers.
Conclusion: As a progress beyond the current state-of-the-art, FORTEe has the ambition to implement pediatric exercise oncology as an evidence-based treatment option for all childhood cancer patients, ultimately integrating it as a standard into clinical practice worldwide.
Trial registration: The FORTEe trial was prospectively registered in the German Clinical Trials Register (DRKS00027978) on 28 January 2022 and on ClinicalTrials.gov (NCT05289739) on 21 March 2022.
Keywords: Cancer-related fatigue; Childhood cancer; Exercise intervention; Pediatric Oncology; Physical activity; Randomized controlled trial; Supportive Care; Training.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The FORTEe study was approved by the Ethics Committee of the Rhineland-Palatinate Chamber of Physicians (application number 2021–15904) on August 4, 2021, with additional approvals from local ethics committees where applicable. Written informed consent was obtained from all participants (or their legal guardians for minors), with documented assent collected from children aged six years and above where possible. Consent for publication: Not applicable. Competing interests: Author M. Polak is affiliated with Nurogames GmbH, which developed and provided the"FORTEe– Get strong"mobile app in the intervention. Author T. Baader is affiliated with Pixformance Sports GmbH, which developed and provided the digital training device (the Pixformance station) used in the intervention. Authors from these companies are not intended to be involved in the data analysis and interpretation mentioned above. The remaining authors declare that they have no competing interests.
Figures
References
-
- Erdmann F, Frederiksen LE, Bonaventure A, Mader L, Hasle H, Robison LL, et al. Childhood cancer: Survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2021;71(Pt B):101733. - PubMed
-
- Vassal G, Schrappe M, Pritchard-Jones K, Arnold F, Basset L, Biondi A, et al. The SIOPE strategic plan: a European cancer plan for children and adolescents. J Cancer Policy. 2016;8:17–32.
-
- Erdmann F, Kaatsch P, Grabow D, C S. German Childhood Cancer Registry - Annual Report 2019 (1980–2018). Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University Mainz; 2020.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

