Acute haemorrhagic necrotizing encephalopathy and inflammatory demyelinating encephalopathy associated with COVID-19 in adults in Southern China
- PMID: 40775306
- PMCID: PMC12330108
- DOI: 10.1186/s12879-025-11404-5
Acute haemorrhagic necrotizing encephalopathy and inflammatory demyelinating encephalopathy associated with COVID-19 in adults in Southern China
Abstract
Background: COVID-19 manifests with diverse systemic symptoms, including central nervous system involvement. Acute necrotizing encephalopathy (ANE), acute hemorrhagic leukoencephalitis (AHLE), and acute disseminated encephalomyelitis (ADEM) exhibit overlapping clinical features, creating diagnostic challenges. This study characterizes COVID-19-associated neuroinflammatory syndromes in patients without apparent respiratory symptoms.
Methods: We conducted a retrospective case series analysis of four patients with confirmed COVID-19 and acute neurological decline. Diagnostic evaluation included brain MRI, cerebrospinal fluid analysis, autoimmune/paraneoplastic antibody panels, and exclusion of alternative etiologies through microbiological/metabolic testing.
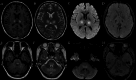
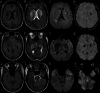
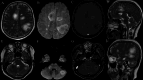
Results: Four cases were identified: two with ANE, one with ADEM, and one with AHLE. All patients tested SARS-CoV-2-positive by RT-PCR despite absent respiratory symptoms. Magnetic resonance imaging revealed characteristic patterns: Symmetric thalamic lesions in ANE (Cases 1-2), hemorrhagic lesions in basal ganglia and bilateral cerebellar hemispheres in AHLE (Case 3), widespread cortical and subcortical demyelination in ADEM (Case 4).
Conclusions: ANE, AHLE, and ADEM are critical neuroinflammatory complications of COVID-19 requiring urgent differentiation. It is imperative to maintain a high level of clinical suspicion when patients present with acute encephalopathy in the absence of respiratory symptoms, as this enables timely intervention.
Keywords: Acute disseminated encephalomyelitis; Acute hemorrhagic leukoencephalitis; Acute necrotizing encephalopathy; COVID-19; Coronavirus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Written informed consent was obtained from the patient for the participation as well as the publication of the case report and any accompanying images. And this study was approved by the ethics committee of South China Agricultural University (ethical number: CR2023-011-01).A copy of the written consent is available upon request from the corresponding author. Consent for publication: Written informed consent for publication of identifying images or other personal or clinical details was obtained from the the participants or parents or legal guardians of any participant under the age of 18. Competing interests: The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

