SREBF1-mediated SND1 transcriptional activation promotes prostate cancer progression via MTDH interaction through the SESN2/AMPK/mTOR axis
- PMID: 40775338
- PMCID: PMC12333249
- DOI: 10.1186/s12967-025-06762-2
SREBF1-mediated SND1 transcriptional activation promotes prostate cancer progression via MTDH interaction through the SESN2/AMPK/mTOR axis
Abstract
Background: Prostate cancer (PCa) is a prevalent cancer and a major cause of cancer-related deaths in men worldwide. Growing evidence indicates that Staphylococcal nuclease and Tudor domain containing 1 (SND1) is a multifunctional protein extensively involved in transcriptional regulation, RNA maturation, post-transcriptional modifications, and other processes. However, previous studies have rarely investigated the function of SND1 as an RNA-binding protein in PCa tumorigenesis.
Methods: The Cancer Genome Atlas and NCBI Gene Expression Omnibus (GEO) databases were used to evaluate SND1 expression levels in PCa. We conducted a series of in vitro and in vivo functional experiments to assess the biological functions of SND1, including cell counting kit-8, colony formation, Transwell and wound-healing assays, and animal experiments in nude mice. Chromatin immunoprecipitation, dual-luciferase reporter assay, and DNA pull-down assay were performed to validate the association between the upstream transcription factor and SND1. Based on mass spectrometry, RNA-seq, and RNA immunoprecipitation (RIP)-seq, we identified the downstream targets of SND1- Sestrin 2 (SESN2), which were validated through qRT-PCR, Western blotting, RIP-qPCR, dual-luciferase reporter assay, and RNA pull-down assay. Finally, a series of functional assays and Western blotting analyses confirmed SESN2 as a downstream target of SND1.
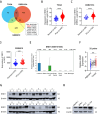
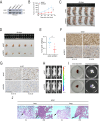
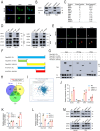
Results: Our research identified that SND1 was significantly elevated in PCa, and knocking down SND1 repressed PCa multiplication and migration. Mechanistically, sterol regulatory element binding transcription factor 1 (SREBF1) bound to the promoter of the SND1 gene and activated its transcription, which subsequently formed a complex with metadherin (MTDH). This complex is directly bound to and degraded SESN2 mRNA, and disruption of this interaction with C26-A6 inhibited MTDH-SND1-mediated SESN2 degradation. Notably, SESN2 expression was inhibited in PCa and may exert tumor-suppressive effects by affecting the AMPK/mTOR signaling pathway. Rescue experiments indicated that knocking down SND1 or MTDH significantly inhibited PCa proliferation and migration, and knocking down SESN2 partially reversed this effect.
Conclusions: Our study reveals SND1 overexpression in PCa, which is transcriptionally activated by SREBF1. Mechanistically, SND1 interacts with MTDH and promotes SESN2 mRNA degradation, modulating PCa progression through the AMPK/mTOR pathway.
Keywords: MTDH; Prostate cancer; SESN2; SND1; mTOR.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experiments conducted in this study were carried out in strict accordance with the Declaration of Helsinki. For studies involving human subjects, written informed consent was obtained from all participants, and their privacy and confidentiality were strictly maintained throughout the research process. Our study received approval from the Institutional Ethics Committee of the First Affiliated Hospital of Medical College, Zhejiang University (Hangzhou, China). The research adhered to the principles of transparency, honesty, and integrity in data collection, analysis, and reporting. Consent for publication: All authors agreed on the manuscript for publication. Conflict of interest: All authors declare no competing interests.
Figures




References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. Cancer J Clin. 2024;74:12–49. - PubMed
-
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in china, 2015. Cancer J Clin. 2016;66:115–32. - PubMed
-
- Galsky MD, Small AC, Tsao C, et al. Clinical development of novel therapeutics for castration-resistant prostate cancer: historic challenges and recent successes. Cancer J Clin. 2012;62:299–308. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

