Sex-based differences in cell migration on aligned topographies
- PMID: 40775422
- PMCID: PMC12331958
- DOI: 10.1038/s41598-025-13450-0
Sex-based differences in cell migration on aligned topographies
Abstract
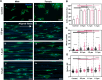
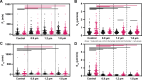
Sexual dimorphism has been observed in many physiological and pathological responses, yet few studies incorporate both female and male experimental groups for preclinical work. For the development of biomaterial devices, in vitro studies are essential for design and optimization, and quantitative comparison of female and male cell migratory behavior is a crucial design consideration. In this work, we thoroughly examined sex-based migration on flat controls and aligned nanofiber scaffolds of various diameters using anomalous and random walk models. Male and female cells exhibited significantly different migration on flat substrates, with female cells having increased speed while male cells had higher persistence. Persistence increased with the introduction of aligned fiber topography for female cells, but only affected male cells on the highest fiber diameter. Speed along the axis of alignment differed between sexes on 1.2 and 1.8 µm fibers. Morphological analysis confirmed cell shape was a function of both sex and fiber size. These results provided critical information regarding sex-based cell migration, highlighting the importance of sex within in vitro studies for clinical device design.
Keywords: Cell migration; Nanofibers; Schwann cells; Sex-based differences.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Psychological interventions for adults who have sexually offended or are at risk of offending.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD007507. doi: 10.1002/14651858.CD007507.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235646 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Gender differences in the context of interventions for improving health literacy in migrants: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2024 Dec 12;12(12):CD013302. doi: 10.1002/14651858.CD013302.pub2. Cochrane Database Syst Rev. 2024. PMID: 39665382
-
Sex and gender as predictors for allograft and patient-relevant outcomes after kidney transplantation.Cochrane Database Syst Rev. 2024 Dec 19;12(12):CD014966. doi: 10.1002/14651858.CD014966.pub2. Cochrane Database Syst Rev. 2024. PMID: 39698949
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
References
-
- Baron-Cohen, S., Knickmeyer, R. C. & Belmonte, M. K. Sex Differences in the brain: Implications for explaining autism. Science310, 819–823. 10.1126/science.1115455 (2005). - PubMed
-
- Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol.16, 626–638. 10.1038/nri.2016.90 (2016). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

