Longitudinal accumulation of glial activation measured by TSPO-PET predicts later brain atrophy in multiple sclerosis
- PMID: 40775641
- PMCID: PMC12333212
- DOI: 10.1186/s12974-025-03519-y
Longitudinal accumulation of glial activation measured by TSPO-PET predicts later brain atrophy in multiple sclerosis
Erratum in
-
Correction: Longitudinal accumulation of glial activation measured by TSPO-PET predicts later brain atrophy in multiple sclerosis.J Neuroinflammation. 2026 Feb 2;23(1):46. doi: 10.1186/s12974-026-03700-x. J Neuroinflammation. 2026. PMID: 41630005 Free PMC article. No abstract available.
Abstract
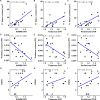
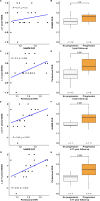
In multiple sclerosis (MS), accumulation of disability is driven by CNS-compartmentalized inflammation. This inflammatory process involves activated microglia and astrocytes, which contribute to neuroaxonal damage which in turn accelerates disease progression. Activated glial cells express 18-kDa translocator protein (TSPO), and TSPO-binding radioligands and positron emission tomography (PET) imaging can be used to quantitate glial activation in vivo. The aim of this study was to evaluate the longitudinal evolution of glial activation in untreated cohorts of relapsing remitting MS (RRMS) and secondary progressive MS (SPMS) patients over one-year follow-up, and to explore how a change in glial activation associates with later imaging and clinical outcomes. Eighteen untreated MS patients (RRMS n = 8, SPMS n = 10) were studied. Expanded disability status scale (EDSS), brain MRI and TSPO-PET scans using [11C]PK11195 were performed at baseline and one year later. Distribution volume ratio (DVR) of [11C]PK11195-binding, and the proportion of TSPO-high voxels at baseline in the normal appearing white matter (NAWM) and other regions of interest were compared to the respective parameters in follow-up scans. Chronic lesions were phenotyped at baseline and at follow-up according to their TSPO-PET-binding patterns, and TSPO-expressing lesions were further characterized using postmortem immunopathological staining. Extended follow-up was obtained after 4-11 years with EDSS available for 18 patients and MR imaging available from 13 patients. TSPO-signal was higher among SPMS compared to RRMS patients at baseline. During one-year follow-up, TSPO uptake remained stable in RRMS patients in all regions of interest. Among the SPMS patients, the proportion of active voxels in the NAWM increased significantly over one-year follow-up. A greater proportion of lesions acquired a rim-active phenotype among SPMS compared to RRMS. According to forward-type stepwise multiple linear regression, change in the proportion of active voxels in the NAWM over one year and baseline body-mass-index were best predictors of later development of brain atrophy (R2 = 0.69). Our study provides novel information about the natural evolution of CNS-compartmentalized inflammation and demonstrates an important link between NAWM TSPO-signal and later adverse outcomes among MS patients, supporting the notion that diffuse glial activation in the NAWM contributes to disease progression.
Keywords: Glia; Microglia; Multiple sclerosis; Positron emission tomography; TSPO.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Human ethics and consent to participate: The study protocol was approved by the Ethics Committee of the Hospital District of Southwest Finland (Dnro:76/180/2008 and Dnro:86/1800/2017). Written informed consent was obtained from all participants according to the principles of the Declaration of Helsinki. The study for postmortem neuropathological staining was approved by the Ethics Committee of the University of Münster (Ref: 2011-153-f-S).
Figures



References
-
- Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis. New England Journal of Medicine. 2018;378:169–80. Available from: https://www.nejm.org/doi/full/10.1056/NEJMra1401483 - DOI - PMC - PubMed
-
- Giovannoni G, Popescu V, Wuerfel J, Hellwig K, Iacobeus E, Jensen MB et al. Smouldering multiple sclerosis: the ‘real MS.’ Ther Adv Neurol Disord. 2022;15. Available from: https://journals.sagepub.com/doi/10.1177/17562864211066751 - DOI - PMC - PubMed
-
- Hamzaoui M, Garcia J, Boffa G, Lazzarotto A, Absinta M, Ricigliano VAG et al. Positron Emission Tomography with [18F]-DPA-714 Unveils a Smoldering Component in Most Multiple Sclerosis Lesions which Drives Disease Progression. Ann Neurol. 2023;94:366–83. Available from: 10.1002/ana.26657 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

