Deconstructing α-Amidoalkyl Sulfones as Dual d-Sulfonyl/ a-Azomethine Synthons: Synthesis of 3-Sulfonylmethylindole Aminals
- PMID: 40775744
- PMCID: PMC12381929
- DOI: 10.1021/acs.joc.5c01392
Deconstructing α-Amidoalkyl Sulfones as Dual d-Sulfonyl/ a-Azomethine Synthons: Synthesis of 3-Sulfonylmethylindole Aminals
Abstract
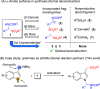
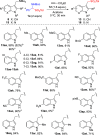
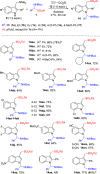
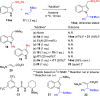
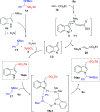
α-Amido sulfones (1) are widely applied as electrophilic aminoalkylation reagents. However, nonproductive sulfinate species are formed alongside. Here, we report that ethyl propiolate-promoted coupling of 1 and gramines provides 3-sulfonylmethylindole aminals II smoothly, thus establishing α-amido sulfones as dual donor/acceptor reagents with full atom incorporation on the target molecule. Simple adjustment of reactants loading allows one to revert the reaction outcome, leading to exclusive formation of 3-sulfonylmethylindoles I instead.
Figures
References
-
- Engberts J. B. F. N., Strating J.. The Mannich condensation of sulfinic acids, aldehyde, and ethyl carbamate II: The use of higher aldehydes. Recl. Trav. Chim. Pays-Bas. 1965;84:942–950. doi: 10.1002/recl.19650840714. - DOI
- Pearson W. H., Lindbeck A. C., Kampf J. W.. Configurational stability of chiral, nonconjugated nitrogen-substituted organolithium compounds generated by tin-lithium exchange of N-[(1-tri-n-butylstannyl)alkyl]imidazolidin-2-ones and -oxazolidin-2-ones. J. Am. Chem. Soc. 1993;115:2622–2636. doi: 10.1021/ja00060a011. - DOI
- Kanazawa A. M., Denis J.-N., Greene A. E.. Highly Stereocontrolled and Efficient Preparation of the Protected, Esterification-Ready Docetaxel (Taxotere) Side Chain. J. Org. Chem. 1994;59:1238–1240. doi: 10.1021/jo00085a004. - DOI
-
- Petrini M.. α-Amido Sulfones as Stable Precursors of Reactive N-Acylimino Derivatives. Chem. Rev. 2005;105:3949–3977. doi: 10.1021/cr050528s. - DOI - PubMed
- Marcantoni E., Palmieri A., Petrini M.. Recent synthetic applications of α-amido sulfones as precursors of N-acylimino derivatives. Org. Chem. Front. 2019;6:2142–2182. doi: 10.1039/C9QO00196D. - DOI
-
-
Selected examples:
- Blay G., Girón R. M., Montesinos-Magraner M., Pedro J. R.. Leaving Group and Regioselectivity Switches in the Aminoalkylation Reaction of Indoles and Related Heterocycles with α-Amido Sulfones. Eur. J. Org. Chem. 2013;2013:3885–3895. doi: 10.1002/ejoc.201300369. - DOI
- Monleón A., Montesinos-Magraner M., Sanz-Marco A., Blay G., Pedro J. R.. Three-Component Synthesis of α-Aminoperoxides Using Primary and Secondary Dialkylzinc Reagents with O2 and α-Amido Sulfones. Org. Lett. 2020;22:5380–5384. doi: 10.1021/acs.orglett.0c01692. - DOI - PubMed
- Gbubele J. D., Misiaszek T., Siczek M., Olszewski T. K.. α-Amido sulphones as useful intermediates in the preparation of C-chiral α-aminophosphonates and α-aminophosphonic acids. Org. Biomol. Chem. 2023;21:6180–6191. doi: 10.1039/D3OB00924F. - DOI - PubMed
-
-
-
Catalytic asymmetric variants (selected examples):
- Gomez-Bengoa E., Linden A., López R., Múgica-Mendiola I., Oiarbide M., Palomo C.. Asymmetric Aza-Henry Reaction Under Phase Transfer Catalysis: An Experimental and Theoretical Study. J. Am. Chem. Soc. 2008;130:7955–7966. doi: 10.1021/ja800253z. - DOI - PubMed
- Park S. Y., Liu Y., Oh J. S., Kweon Y. K., Jeong Y. B., Duan M., Tan Y., Lee J.-W., Yan H., Song C. E.. Asymmetric Aminalization via Cation-Binding Catalysis. Chem. - Eur. J. 2018;24:1020–1025. doi: 10.1002/chem.201703800. - DOI - PubMed
- Serusi L., Palombi L., Pierri G., Di Mola A., Massa A.. Asymmetric Cascade Aza-Henry/Lactamization Reaction in the Highly Enantioselective Organocatalytic Synthesis of 3-(Nitromethyl)isoindolin-1-ones from α-Amido Sulfones. J. Org. Chem. 2022;87:8420–8428. doi: 10.1021/acs.joc.2c00518. - DOI - PMC - PubMed
-
LinkOut - more resources
Full Text Sources

