Evaluation and study of adverse reactions to imiglucerase based on the FAERS database
- PMID: 40775785
- PMCID: PMC12329963
- DOI: 10.1186/s13023-025-03934-7
Evaluation and study of adverse reactions to imiglucerase based on the FAERS database
Abstract
Objective: This study aims to evaluate the adverse drug reactions associated with imiglucerase in the treatment of Gaucher disease by analyzing data from the FDA Adverse Event Reporting System (FAERS) database.
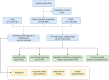
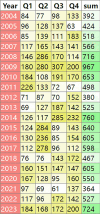
Methods: A comprehensive analysis was conducted on 166,800,135 adverse event reports from the FAERS database, covering the period from the first quarter of 2004 to the fourth quarter of 2023. The data were processed using R software and analyzed using multiple disproportionality methods, including Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-Item Gamma Poisson Shrinker (MGPS). These methodologies were applied to identify significant adverse reaction signals across various System Organ Classes (SOCs) and Preferred Terms (PTs).
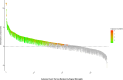
Results: The analysis revealed significant adverse reaction signals in multiple SOCs, including general disorders and administration site conditions, injury, poisoning and procedural complications, infections and infestations, and nervous system disorders. Notably, general disorders and injury-related conditions had the highest number of reports. At the PT level, the term "Gaucher disease" yielded the highest statistical signal. This was identified as a critical reporting artifact, likely representing perceived treatment failure or disease progression, rather than a true adverse reaction. After accounting for this artifact, other significant adverse event signals included increased chitotriosidase, elevated acid phosphatase, and bone infarction, with musculoskeletal and connective tissue disorders being a key area of concern. A comparative analysis against other Gaucher therapies suggests this strong skeletal signal likely reflects confounding by indication rather than a drug-specific risk.
Conclusion: The findings underscore the importance of ongoing pharmacovigilance to monitor the safety of imiglucerase, especially among vulnerable populations such as pregnant women, long-term users, and those with comorbid hepatobiliary or skeletal conditions.
Keywords: Adverse drug reactions; FAERS database; Gaucher disease; Imiglucerase; Pharmacovigilance.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that there are no conflicts of interest regarding this study.
Figures
Similar articles
-
Adverse drug reactions related to methotrexate: a real-world pharmacovigilance study using the FAERS database from 2004 to 2024.Front Immunol. 2025 Jun 4;16:1586361. doi: 10.3389/fimmu.2025.1586361. eCollection 2025. Front Immunol. 2025. PMID: 40534848 Free PMC article. Review.
-
Safety profile of ramelteon from the perspective of physicians and pharmacists: a 20-year real-world pharmacovigilance analysis based on the FAERS database.BMC Psychiatry. 2025 Jul 7;25(1):683. doi: 10.1186/s12888-025-07127-1. BMC Psychiatry. 2025. PMID: 40624487 Free PMC article.
-
Sex differences in drug-induced osteoporosis: a pharmacovigilance study based on the FAERS database.Front Public Health. 2025 Jul 24;13:1630412. doi: 10.3389/fpubh.2025.1630412. eCollection 2025. Front Public Health. 2025. PMID: 40777658 Free PMC article.
-
A real-world pharmacovigilance study of Certolizumab pegol based on FAERS database.Sci Rep. 2025 Aug 5;15(1):28529. doi: 10.1038/s41598-025-13502-5. Sci Rep. 2025. PMID: 40764349 Free PMC article.
-
Enzyme replacement and substrate reduction therapy for Gaucher disease.Cochrane Database Syst Rev. 2015 Mar 27;2015(3):CD010324. doi: 10.1002/14651858.CD010324.pub2. Cochrane Database Syst Rev. 2015. PMID: 25812601 Free PMC article.
References
-
- Charrow J, Andersson HC, Kaplan P, Kolodny EH, Mistry P, Pastores G, Rosenbloom BE, Scott CR, Wappner RS, Weinreb NJ, et al. The Gaucher registry: demographics and disease characteristics of 1698 patients with Gaucher disease. Arch Intern Med. 2000;160(18):2835–43. - PubMed
-
- Nalysnyk L, Rotella P, Simeone JC, Hamed A, Weinreb N. Gaucher disease epidemiology and natural history: a comprehensive review of the literature. Hematology. 2017;22(2):65–73. - PubMed
-
- Granovsky-Grisaru S, Belmatoug N, vom Dahl S, Mengel E, Morris E, Zimran A. The management of pregnancy in Gaucher disease. Eur J Obstet Gynecol Reprod Biol. 2011;156(1):3–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

