Plasma phosphorylated tau 217 and amyloid‑β 42/40 for amyloid risk in subgroups
- PMID: 40775788
- PMCID: PMC12329931
- DOI: 10.1186/s13195-025-01826-3
Plasma phosphorylated tau 217 and amyloid‑β 42/40 for amyloid risk in subgroups
Abstract
Background: Alzheimer's disease (AD) is characterized by the accumulation of amyloid-β (Aβ) pathology. Recently, plasma biomarkers, particularly p-tau217, have emerged as promising tools for early diagnosis and risk stratification. In this retrospective study, we evaluated the diagnostic performance of p-tau217 combined with other plasma biomarkers in distinguishing Aβ Positron emission tomography (PET) positivity in cognitively unimpaired (CU) and cognitively impaired (CI) individuals across diverse clinical subgroups.
Methods: We analyzed 2,497 participants from the Korea-Registries to Overcome dementia and Accelerate Dementia (K-ROAD) cohort, including 636 CU and 1,971 CI individuals. Plasma p-tau217 was measured using both SIngle MOlecule Array (SIMOA) and Meso Scale Discovery (MSD) assays, alongside Aβ42/40, Glial fibrillary acidic protein (GFAP), and Neurofilament light chain (NfL). We assessed the diagnostic performance of biomarker combinations for Aβ PET positivity through the area under the receiver operating characteristic curve (AUC), Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC), and performed subgroup analyses based on age, sex, body mass index (BMI), and Apolipoprotein E (APOE) ε4 status. To assess applicability, we stratified the cohort by recruitment site into a development set (Samsung Medical Center, n = 1,545) and a validation set (other centers, n = 952).
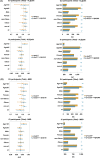
Results: In CU individuals from the development cohort, the combination of p-tau217 and Aβ42/40 significantly improved diagnostic accuracy (AUC: ALZpath 0.937 vs. 0.905, MSD 0.901 vs. 0.861; p < 0.05, DeLong test; 95% CIs) and model fit (AIC /BIC, p < 0.001) compared to p-tau217 alone. In contrast, in CI individuals, the combination provided only modest improvements in model fit without significantly enhancing AUC. GFAP and NfL did not contribute significantly to amyloid detection in either group. These findings were successfully validated in an independent cohort from other centers. Subgroup analyses in CU individuals showed the greatest improvements in older adults, females, and APOE4 non-carriers, regardless of obesity status. In CI individuals, the combination had no significant impact on AUC except in males, where a small but significant increase was observed (p = 0.002).
Conclusion: Combining p-tau217 with Aβ42/40 enhances amyloid detection in CU individuals, improving both diagnostic accuracy and model fit, whereas its impact in CI individuals is limited. These results highlight the potential of plasma biomarker combinations for refining early AD diagnostics and individualized risk assessment.
Keywords: Alzheimer’s disease; Aβ42/40; Diagnostic accuracy; Plasma biomarkers; p-tau217.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The institutional review board of Samsung Medical Center (No. 2021-02-135) approved this study. All participants provided informed consent to participate in the study, and the data were collected in accordance with the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: Henrik Zetterberg has served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave. He has delivered lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche. He is also a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is part of the GU Ventures Incubator Program (outside the submitted work). Kaj Blennow has served as a consultant and advisory board member for Abbvie, AC Immune, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers. He has served on data monitoring committees for Julius Clinical and Novartis. He has also delivered lectures, produced educational materials, and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai, and Roche Diagnostics. Additionally, he is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is part of the GU Ventures Incubator Program, outside the work presented in this paper. All other authors declare no conflicts of interest. Theresa A. Day is an employee and minor stockholder of Eli Lilly and Company.
Figures

References
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. 10.1126/science.1072994. - PubMed
MeSH terms
Substances
Grants and funding
- #2023-00356, #2022-01018, #2019-02397/Swedish Research Council
- 101053962/European Union's Horizon Europe research and innovation program
- ALFGBG-71320/Swedish State Support for Clinical Research
- 201809-2016862/Alzheimer Drug Discovery Foundation (ADDF), USA
- ADSF-21-831376-C, ADSF-21-831381-C, ADSF-21-831377-C, ADSF-24-1284328-C/AD Strategic Fund and the Alzheimer's Association
- FO2022-0270/Hjärnfonden, Sweden
- #FO2017-0243, #ALZ2022-0006/Hjärnfonden, Sweden
- 860197/EU Horizon 2020 (Marie Skłodowska-Curie grant - MIRIADE)
- JPND2021-00694/European Union Joint Programme- Neurodegenerative Disease Research (JPND)
- UKDRI-1003/UK Dementia Research Institute at UCL
- #AF-930351, #AF-939721, #AF-968270, #AF-994551/Swedish Alzheimer Foundation
- #ALFGBG-715986, #ALFGBG-965240/Swedish State (ALF agreement between Government and County Councils)
- JPND2019-466-236/European Union Joint Program- Neurodegenerative Disorders (JPND)
- ZEN-21-848495/Alzheimer's Association (Zenith Award)
- SG-23-1038904 QC/Alzheimer's Association 2022-2025 Grant
- 2024-R001/Korean Dementia Association
- RS-2020-KH106434/Ministry of Health & Welfare and Ministry of Science and ICT, Republic of Korea (via KDRC)
- RS-2019-NR040057/National Research Foundation of Korea (NRF), Korea government (MSIT)
- RS-2021-II212068/Institute of Information & Communications Technology Planning & Evaluation (IITP), MSIT
- #SMX1250081/Samsung Medical Center, Future Medicine 20*30 Project
- 2024-ER1003-01/Korea National Institute of Health
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

