Ten-Year Comparison of Fungicide Sensitivity and Mycotoxin Production of Fusarium Head Blight Isolates from Korea
- PMID: 40776548
- PMCID: PMC12332407
- DOI: 10.5423/PPJ.OA.05.2025.0068
Ten-Year Comparison of Fungicide Sensitivity and Mycotoxin Production of Fusarium Head Blight Isolates from Korea
Abstract
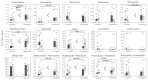
Fusarium head blight (FHB) is an important disease reducing yield and quality of wheat and barley. To study changes in fungicide efficacy over time, 161 FHB isolates (F. asiaticum and F. graminearum) were obtained from infected wheat and barley in the Jeolla provinces of the Republic of Korea from 2010-2013 and 2020-2023. Over 10 years, FHB fungi developed resistance to demethylation inhibitors (DMIs), methyl benzimidazole carbamates (MBCs), and phthalimides, with few exceptions. Also, no significant resistance against succinate dehydrogenase inhibitors (SDHI) and quinoneoutside inhibitors (QoI) was observed, but sensitivity to phenylpyrrole (PP) increased. Mycotoxin production by four representative isolates of both species indicated that higher doses of DMI, DMI + DMI, MBC, MBC + DMI, and PP controlled trichothecenes, whereas zearalenone was controlled only by SDHI. QoI, QoI + DMI, and phthalimide did not control mycotoxin production in either species. Despite resistance development, DMI, MBC, and PP can still be used to control FHB and mycotoxins in wheat and barley in Korea with close monitoring of resistance.
Keywords: cereals; effective concentration 50%; scab; toxin.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



References
-
- Ahn S., Kim M., Lim J. Y., Choi G. J., Seo J.-A. Characterization of Fusarium asiaticum and F. graminearum isolates from gramineous weeds in the proximity of rice fields in Korea. Plant Pathol. 2022;71:1164–1173.
-
- Andrade S. M. P., Augusti G. R., Paiva G. F., Feksa H. R., Tessmann D. J., Machado F. J., Mizubuti E. S. G., Del Ponte E. M. Phenotypic and molecular characterization of the resistance to azoxystrobin and pyraclostrobin in Fusarium graminearum populations from Brazil. Plant Pathol. 2022;71:1152–1163.
-
- Baek J., Nah J.-Y., Lee M.-J., Lim S.-B., Choi J.-H., Jang J. Y., Lee T., Choi H.-W., Kim J. Change in the sensitivity to propiconazole of Fusarium graminearum species complex causing head blight of barley and wheat in Jeolla Province. Korean J. Mycol. 2022;50:281–289. (in Korean)
-
- Barro J. P., Santana F. M., Tibola C. S., Machado F. J., Schipanski C. A., Chagas D. F., Guterres C. W., Casarotto G., Capitanio C. G., Dallagnol L. J., Kuhnem P., Feksa H. R., Venancio W. S., Del Ponte E. M. Comparison of single- or multi-active ingredient fungicides for controlling Fusarium head blight and deoxynivalenol in Brazilian wheat. Crop Prot. 2023;174:106402.
-
- Becher R., Hettwer U., Karlovsky P., Deising H. B., Wirsel S. G. R. Adaptation of Fusarium graminearum to tebuconazole yielded descendants diverging for levels of fitness, fungicide resistance, virulence, and mycotoxin production. Phytopathology. 2010;100:444–453. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

