Assessing laboratory specimen losses for the city of Johannesburg, South Africa
- PMID: 40776713
- PMCID: PMC12339880
- DOI: 10.4102/phcfm.v17i1.4907
Assessing laboratory specimen losses for the city of Johannesburg, South Africa
Abstract
Background: Specimen losses across the pathology value chain (PVC) result in missed diagnostic opportunities. It is difficult to fully assess these due to the current paper-based systems, with tracking of specimens only possible on the laboratory information system (LIS).
Aim: This study aimed to assess specimen losses using the paper-based register.
Setting: Randomly selected Primary health care (PHC) facilities, City of Johannesburg, South Africa.
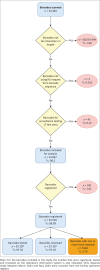
Methods: The retrospective descriptive study design was used to scan 1,000 barcodes from facilities in sub-districts A to G. Data was limited to barcodes from the request form and excluded surveillance testing. Matching data from the laboratory repository was extracted. PVC losses were assessed by determining the percentage of scanned barcodes that had a registered, tested, reviewed and/or rejected date. The analysis was stratified according to sub-district, health facility type and test code.
Results: The dataset analysed included 33 867 barcodes with 121 697 test codes, equating to 3.59 tests per barcode. Matching registered, tested and reviewed dates were detected for 33 107 (97.76%) barcodes. In total, a rejection for one or more test codes was detected for 1,961 barcodes (5.79%). At the sub-district level, between 95.95% (D) and 98.90% (E) of barcodes were reviewed. The rejection rate ranged from 3.27% (F) to 10.93% (D). For community health centres and clinics, 97.37% and 97.97% of the barcodes had a matching reviewed date.
Conclusion: PVC losses reported were 4.05%, excluding rejections (5.79%), with slightly higher levels noted at the sub-district level. Contribution: The continuous audit of PVC losses is recommended.
Keywords: laboratory information system; losses; pathology value chain; primary health care; rejection; repository.
Conflict of interest statement
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Figures
Similar articles
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Variation within and between digital pathology and light microscopy for the diagnosis of histopathology slides: blinded crossover comparison study.Health Technol Assess. 2025 Jul;29(30):1-75. doi: 10.3310/SPLK4325. Health Technol Assess. 2025. PMID: 40654002 Free PMC article.
-
123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma.Cochrane Database Syst Rev. 2015 Sep 29;2015(9):CD009263. doi: 10.1002/14651858.CD009263.pub2. Cochrane Database Syst Rev. 2015. PMID: 26417712 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
References
-
- Sharaki O, Abouzeid A, Hossam N, Elsherif Y. Self-assessment of pre, intra and post analytical errors of urine analysis in Clinical Chemistry Laboratory of Alexandria Main University Hospital. Saudi J Health Sci. 2014;3(2):96–102. 10.4103/2278-0521.134863 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources