Liver metastasis of colorectal cancer: Mechanism and clinical therapy (Review)
- PMID: 40776741
- PMCID: PMC12351159
- DOI: 10.3892/or.2025.8963
Liver metastasis of colorectal cancer: Mechanism and clinical therapy (Review)
Abstract
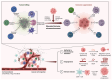
Liver metastasis is a common complication in colorectal cancer (CRC), with its presence and progression significantly shortening patient survival. Therefore, a deeper understanding of the underlying mechanisms driving liver metastasis in CRC is essential to identify more effective and actionable therapeutic targets and improve prognosis. Liver metastasis in CRC is a multifaceted and dynamic process. Tumor cells with invasive properties communicate with the surrounding microenvironment through mechanisms such as immune checkpoint molecules and cytokines, thereby establishing a supportive niche for their colonization and proliferation. Moreover, suppressive immune cells may enhance the invasiveness of tumor cells. The interplay between tumor cells and the microenvironment is an interdependent process. Targeting these interactions offers promising potential for novel therapeutic strategies. The present review outlined mechanisms of colorectal cancer liver metastasis, emphasizing the immune microenvironment's role, current treatment approaches, and future development prospects.
Keywords: colorectal cancer; immune microenvironment; liver metastasis; mechanism.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

