Knockdown of LINC00467 inhibits gastric cancer progression by modulating the sequestration of miR-141-3p
- PMID: 40776904
- PMCID: PMC12329486
- DOI: 10.3892/ol.2025.15205
Knockdown of LINC00467 inhibits gastric cancer progression by modulating the sequestration of miR-141-3p
Abstract
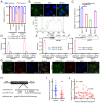
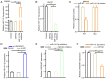
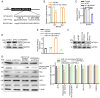
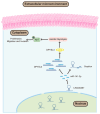
Gastric cancer (GC) is one of the most common malignancies globally, with notable morbidity and mortality rates. Despite advances in surgical techniques and adjuvant therapies, recurrence and metastasis remain major challenges, highlighting the need for novel biomarkers and therapeutic targets. Long non-coding RNAs (lncRNAs) have emerged as key regulators in various types of cancer, including GC, which can influence tumor progression through diverse mechanisms. LINC00467, in particular, has been implicated in non-small cell lung cancer, hepatocellular carcinoma and colorectal cancer, but the role of LINC00467 in GC remains poorly understood. The present study aimed to elucidate the role of LINC00467 in GC progression by investigating its expression patterns, functional impact on cellular behaviors and underlying molecular mechanisms. The expression levels of LINC00467 were evaluated in the GEPIA database of human gastric cancer samples, which demonstrated LINC00467 upregulation in 60 tumor tissue samples from patients with GC compared with that of paired para-cancerous control tissues. Functionally, LINC00467 promoted glycolysis in GC cells and enhanced their proliferative, migratory and invasive activities. From a mechanistic perspective, LINC00467 was able to bind to microRNA (miR)-141-3p in GC cells and a negative correlation between miR-141-3p and LINC00467 expression was observed in GC tissue samples. Inhibition of miR-141-3p partially reversed the effects of LINC00467 knockdown on GC cell malignancy and LINC00467 was further found to control the expression of the miR-141-3p target gene dihydropyriminidase-like 3 (DPYSL3) in GC cells. Furthermore, lactate accumulation from glycolysis activated the AKT signaling pathway to promote the transcriptional expression of LINC00467 in GC cells and led to persistent glycolysis and GC cell invasion. The present study findings suggested that LINC00467 potentially controls GC progression via regulation of the miR-141-3p/DPYSL3 pathway.
Keywords: LINC00467; dihydropyriminidase-like 3; gastric cancer progression; microRNA-141-3p.
Copyright: © 2025 Ju et al.
Conflict of interest statement
The authors declare that the they have no competing interests.
Figures





Similar articles
-
miRNA-223-3p regulates ECT2 to promote proliferation, invasion, and metastasis of gastric cancer through the Wnt/β-catenin signaling pathway.J Cancer Res Clin Oncol. 2023 Jan;149(1):121-134. doi: 10.1007/s00432-022-04453-9. Epub 2022 Nov 10. J Cancer Res Clin Oncol. 2023. PMID: 36355210 Free PMC article.
-
IL-21R functions as an oncogenic factor and is regulated by the lncRNA MALAT1/miR-125a-3p axis in gastric cancer.Int J Oncol. 2019 Jan;54(1):7-16. doi: 10.3892/ijo.2018.4612. Epub 2018 Oct 31. Int J Oncol. 2019. PMID: 30387833 Free PMC article.
-
SPINT1-AS1 promotes oxidative damage and apoptosis of gastric cancer cells via the miR-656-3p/PLCXD3 axis.Discov Oncol. 2025 Aug 9;16(1):1515. doi: 10.1007/s12672-025-03195-7. Discov Oncol. 2025. PMID: 40783674 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
