Cardiovascular-Kidney-Metabolic Effects: Steroidal and Nonsteroidal Mineralocorticoid Receptor Antagonists
- PMID: 40776944
- PMCID: PMC12326440
- DOI: 10.31083/RCM38690
Cardiovascular-Kidney-Metabolic Effects: Steroidal and Nonsteroidal Mineralocorticoid Receptor Antagonists
Abstract
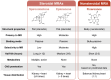
Cardiovascular (CV)-kidney-metabolic (CKM) syndrome is a complex disorder characterized by the co-occurrence of CV risk factors, including chronic kidney disease (CKD), hypertension, and metabolic dysfunction, which creates a vicious cycle where one factor negatively impacts the others, ultimately leading to poor overall CV and kidney outcomes. Overactivation of the mineralocorticoid receptor, through binding with aldosterone and ligand-independent mechanisms, is implicated in the pathogenesis of CKM; mineralocorticoid receptor antagonists (MRAs) can block this interaction. Steroidal MRAs are currently recommended for people with heart failure (HF) with reduced ejection fraction and hypertension; however, the role of nonsteroidal MRAs in CKM is evolving. Indeed, steroidal MRAs have demonstrated efficacy against composite CV-related mortality and hospitalization, elevated systolic blood pressure, and hospitalizations for worsening HF in clinical trials of individuals with HF, CKD, and treatment-resistant hypertension. Moreover, the nonsteroidal MRA finerenone has demonstrated risk reductions for composite CV-related outcomes and CKD progression in patients with HF with mildly reduced or preserved ejection fraction and people with CKD associated with type 2 diabetes. Ongoing phase 3 trials are evaluating the efficacy and safety of nonsteroidal MRAs in individuals with HF and reduced ejection fraction, as well as those with mildly reduced or preserved ejection fraction, potentially expanding their role in managing CKM conditions. This review examines current clinical evidence for the use of MRAs in people with CKM syndrome.
Keywords: cardio-kidney-metabolic syndrome; heart failure; mineralocorticoid receptor antagonists.
Copyright: © 2025 The Author(s). Published by IMR Press.
Conflict of interest statement
Biykem Bozkurt reports consultancy with Abbott, Abiomed, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cardurion, Cytokinetics, Daiichi Sankyo, Johnson & Johnson, Lantheus, Liva Nova, Merck, Regeneron, Renovacor, Respicardia/Zoll, Roche, Sanofi-Aventis, and Vifor. James L. Januzzi has received consulting fees or advisory and funding grants from Roche Diagnostics, Siemens Diagnostics, Abbott Diagnostics, and Merck. Shweta Bansal has received research funding from the National Institutes of Health, Bayer, Novo Nordisk, and Boehringer Ingelheim; royalties from UpToDate; and she serves as faculty speaker for Home Dialysis University and in the advisory boards for Calliditas Therapeutics, Novartis, Vera, and Travere Therapeutics. However, these companies had no role in the handling or conduct of the study.
Figures
References
-
- Roth GA, Dorsey H, Decleene N, Razo C, Stark B, Johnson C. The global burden of heart failure: a systematic analysis for the Global Burden of Disease Study 2021. European Heart Journal . 2023;44:876. doi: 10.1093/eurheartj/ehad655.876. - DOI
-
- Ndumele CE, Neeland IJ, Tuttle KR, Chow SL, Mathew RO, Khan SS, et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement From the American Heart Association. Circulation . 2023;148:1636–1664. doi: 10.1161/CIR.0000000000001186. - DOI - PubMed
-
- Ostrominski JW, Claggett BL, Miao ZM, Mc Causland FR, Anand IS, Desai AS, et al. Cardiovascular-Kidney-Metabolic Overlap in Heart Failure With Mildly Reduced or Preserved Ejection Fraction: A Trial-Level Analysis. Journal of the American College of Cardiology . 2024;84:223–228. doi: 10.1016/j.jacc.2024.05.005. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous