Stented Biological Prosthesis Versus Mitral Allograft in Surgical Treatment of Tricuspid Valve Infective Endocarditis
- PMID: 40776959
- PMCID: PMC12326450
- DOI: 10.31083/RCM37204
Stented Biological Prosthesis Versus Mitral Allograft in Surgical Treatment of Tricuspid Valve Infective Endocarditis
Abstract
Background: The prevalence of tricuspid valve (TV) infective endocarditis (IE) continues to increase among patients with drug addictions and chronic vascular access or cardiac electronic devices. Moreover, long-term mortality and morbidity following surgery with conventional prostheses remain high. Allografts may represent a suitable alternative in tricuspid surgery. This study aimed to compare outcomes between stented biological valves and mitral allografts (MAs) for tricuspid valve replacement (TVR).
Methods: A total of 54 patients with IE underwent TVR using either a stented bioprosthesis (B) or MA between January 2016 and July 2024. Clinical and echocardiographic data were analyzed in accordance with the Tricuspid-Valve Academic Research Consortium (T-VARC) criteria. Early safety, clinical efficacy, and time-to-event survival were compared between the two equal B and MA groups.
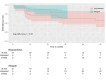
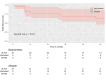
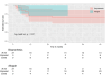
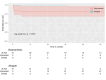
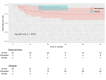
Results: There were no in-hospital or 30-day mortality, nor cardiac, cerebral, and wound complications in either group. The peak and mean pressure gradient (PG) on TV after surgery were 9.2 (6.5-12.0) and 4.0 (3.2-6.0) mmHg in the B group versus 6.0 (4.5-7.5) and 3.0 (2.0-4.0) mmHg in the MA group (p < 0.001). A T-VARC-adjusted analysis demonstrated superior freedom from cardiovascular mortality, recurrent IE, reoperation, and permanent pacemaker implantation (PPI) in the MA group 2 years after operation. Kaplan-Meier analysis revealed significantly higher freedom from cardiovascular mortality in the MA group (100% vs. 81.5%, 77.8%, 77.8%, 69.6% respectively (log-rank test, p = 0.011) at 12-, 18-, 24-, 36-months, and freedom from PPI (100% vs. 81% at all time intervals) (log-rank test, p = 0.021).
Conclusion: Application of contemporary endpoint criteria demonstrated superior outcomes with MA, including lower cardiovascular mortality, reduced PPI, fewer recurrent endocarditis, decreased reoperations, cardiac hospitalizations, alongside improved patient-reported outcomes. Time-to-event analysis demonstrated benefits in cardiovascular survival and PPI avoidance with allografts. Mitral allograft may be a preferable alternative valve substitute for TVR in patients with IE.
Clinical trial registration: ClinicalTrials.gov ID: NCT06591000, https://clinicaltrials.gov/study/NCT06591000?term=NCT06591000&rank=1, registration date: September 19, 2024.
Keywords: allograft; endocarditis; tricuspid valve replacement.
Copyright: © 2025 The Author(s). Published by IMR Press.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Iftikhar SF, Ahmad F. Tricuspid Valve Endocarditis . StatPearls Publishing: Treasure Island; 2025. - PubMed
-
- Slaughter MS, Badhwar V, Ising M, Ganzel BL, Sell-Dottin K, Jawitz OK, et al. Optimum surgical treatment for tricuspid valve infective endocarditis: An analysis of the Society of Thoracic Surgeons national database. The Journal of Thoracic and Cardiovascular Surgery . 2021;161:1227–1235.e1. doi: 10.1016/j.jtcvs.2019.10.124. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

