Impact of maternal microbiota imbalance during pregnancy on fetal cerebral neurodevelopment: Is there a link to certain autistic disorders?
- PMID: 40776983
- PMCID: PMC12329290
- DOI: 10.1016/j.bbih.2025.101074
Impact of maternal microbiota imbalance during pregnancy on fetal cerebral neurodevelopment: Is there a link to certain autistic disorders?
Abstract
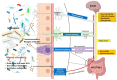
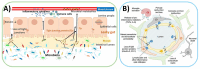
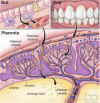
Autism spectrum disorder (ASD) is a neurodevelopmental disorder associated with a heterogeneous group of psychiatric disorders primarily characterized by impairments in communication and social interactions. ASD typically emerges around 18 months of age. While no single etiology has been identified, theories suggest a combination of genetic predisposition and environmental factors, such as exposure to toxics, viral infections, and neuroinflammatory processes. However, the mechanisms linking environmental risk factors to ASD remain poorly understood, particularly during the prenatal period. Among the various hypotheses, the gut microbiota has been proposed as a potential modulator of nervous system development and function. During pregnancy, the maternal microbiota could trigger gestational inflammatory responses, leading to maternal immune activation (MIA). These deleterious processes could play a causal role in the etiopathogenesis of neurodevelopmental disorders in offspring. The gut microbiota could be the missing link between genetic susceptibility and environmental exposures in certain forms of ASD. The gut microbiome induces the production of microbiota-derived signaling metabolites, immune mediators, gut hormones, and neural signaling via the spinal cord and vagus nerve. This review synthesizes the current knowledge from preclinical rodent models and human studies that investigate the impact of the maternal gut microbiota during pregnancy on ASD risk in offspring. Additionally, the potential roles of the maternal oral and vaginal microbiota are also discussed in the context of this maternal-offspring pairing. Finally, we examine how restoring maternal microbiome balance, through interventions such as pre/probiotics, may help reduce the perinatal risk of certain ASD by positively influencing prenatal environmental factors.
Keywords: Fetal neurodevelopment; MIA; Maternal microbiome.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Aagaard K., Møllegaard Jepsen J.R., Sevelsted A., Horner D., Vinding R., Rosenberg J.B., Brustad N., Eliasen A., Mohammadzadeh P., Følsgaard N., Hernández-Lorca M., Fagerlund B., Glenthøj B.Y., Rasmussen M.A., Bilenberg N., Stokholm J., Bønnelykke K., Ebdrup B.H., Chawes B. High-dose vitamin D3 supplementation in pregnancy and risk of neurodevelopmental disorders in the children at age 10: a randomized clinical trial. Am. J. Clin. Nutr. 2024;119:362–370. - PubMed
-
- Abdalkareem Jasim S., Jade Catalan Opulencia M., Alexis Ramírez-Coronel A., Kamal Abdelbasset W., Hasan Abed M., Markov A., Raheem Lateef Al-Awsi G., Azamatovich Shamsiev J., Thaeer Hammid A., Nader Shalaby M., Karampoor S., Mirzaei R. The emerging role of microbiota-derived short-chain fatty acids in immunometabolism. Int. Immunopharmacol. 2022;110 - PubMed
-
- Al-Ewaidat O.A., Gogia S., Naffaa M.M. Omega-3 PUFAs: a multifaceted approach to lifespan brain health, neurodevelopment, and precision therapeutics with implications for ASD, ADHD, and cognitive function. Dis. Res. 2025;4:1–23.
Publication types
LinkOut - more resources
Full Text Sources

