Dissecting the intratumoral microbiome landscape in lung cancer
- PMID: 40777004
- PMCID: PMC12328415
- DOI: 10.3389/fimmu.2025.1614731
Dissecting the intratumoral microbiome landscape in lung cancer
Abstract
The discovery of microbial communities residing within tumors has unveiled a new dimension of cancer biology. In lung cancer, the intratumoral microbiome-comprising bacteria, fungi, and viruses-has emerged as a critical modulator of tumorigenesis, immune evasion, therapeutic response, and metastasis. This review comprehensively examines the landscape of the lung tumor microbiota, highlighting its mechanistic roles in shaping the tumor microenvironment, altering host immune responses, and reprogramming of cancer metabolism. We discuss the influence of specific microbial taxa on immunotherapeutic efficacy, including their interplay with immune checkpoints and pro-inflammatory signaling pathways. Moreover, we evaluate current evidence linking microbial signatures for diagnostic and prognostic applications, emphasizing their potential in biomarker discovery and precision oncology. By integrating findings from molecular epidemiology, multi-omics profiling, and preclinical models, this review provides a translational framework for leveraging the tumor-resident microbiota as both a within tumors, we may develop new microbiome-based strategies. These strategies could improve treatment outcomes and help overcome resistance to immunotherapy.
Keywords: cancer therapy; intratumoral microbiome; lung cancer; microbial interactions; tumor microenvironment.
Copyright © 2025 Zhao, Yang, Wu and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

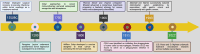
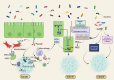
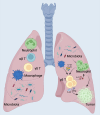

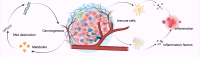
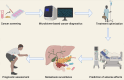
References
-
- Joksić I, Zmrzljak UP, Ninić A, Ratković T, Munjas J. Genetic predisposition for ovarian cancer development. Arch Pharm. (2025) 75:32–43. doi: 10.5937/arhfarm75-55120 - DOI
-
- Kiemeney LA, Rothman N, Koutros S, Prokunina-Olsson L, Verhaegh G, Vermeulen SH. Genetic predisposition to bladder cancer. In: Biology of Bladder Cancer: From Molecular Insights to Clinical Strategies. Springer; (2025). p. 23–55.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

