Non-invasive physical plasma activates stimulator of interferon genes pathway in triple negative breast cancer and is associated with increased host immune response
- PMID: 40777006
- PMCID: PMC12328447
- DOI: 10.3389/fimmu.2025.1631530
Non-invasive physical plasma activates stimulator of interferon genes pathway in triple negative breast cancer and is associated with increased host immune response
Abstract
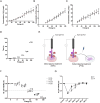
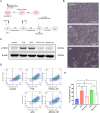
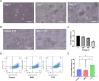
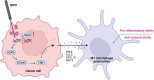
Triple-negative breast cancer (TNBC), characterized by the absence of ER, PR, and HER2 receptors, remains one of the most aggressive breast cancer subtypes, with limited therapeutic options and a high relapse rate. While immune checkpoint inhibitors (ICIs) have shown promise by leveraging TNBC's immunogenic profile, their use is often accompanied by significant toxicity, necessitating the development of safer immunomodulatory strategies. Non-invasive physical plasma (NIPP), a novel low thermal plasma technology that can be generated using various gases, including argon, and producing reactive oxygen and nitrogen species (RONS), has emerged as a potential alternative. This study investigates the capacity of direct (argon plasma devitalization, APD) and indirect (plasma-treated solution, PTS) plasma modalities to induce cytotoxicity and activate immune signaling via the stimulator of interferon genes (STING) pathway in TNBC. Dose-dependent RONS generation by APD and PTS correlated with reduced viability and apoptosis induction in MDA-MB-231 TNBC cells. Both plasma modalities caused DNA damage and upregulated key proteins in the STING pathway, including γ-H2AX, p-STING, and p-TBK1, with sustained activation observed up to 24 hours post-treatment. Furthermore, STING-dependent transcription of IFN-β and interferon-stimulated genes (ISGs) confirmed the immunogenic potential of NIPP. Conditioned media from plasma-treated TNBC cells induced M1 polarization in THP-1-derived macrophages, an effect significantly reduced upon specific STING inhibition with H-151. The immunomodulatory effects of NIPP were validated in patient-derived TNBC organoids, where plasma treatment disrupted organoid structure, reduced viability, and promoted M1 macrophage polarization. Collectively, these findings highlight the dual cytotoxic and immunostimulatory potential of NIPP in TNBC through STING pathway activation, claiming it as a promising, low-toxicity component in combination with conventional immunotherapy.
Keywords: argon plasma devitalization (APD); cold atmospheric plasma (CAP); non-invasive physical plasma (NIPP); plasma-treated solution (PTS); triple negative breast cancer.
Copyright © 2025 Wang, Arnholdt, Koch, Enderle, Hahn, Brucker and Weiss.
Conflict of interest statement
ME is employee of Erbe Elektromedizin GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
MYC controls STING levels to downregulate inflammatory signaling in breast cancer cells upon DNA damage.J Biol Chem. 2025 Jun;301(6):108560. doi: 10.1016/j.jbc.2025.108560. Epub 2025 Apr 29. J Biol Chem. 2025. PMID: 40311680 Free PMC article.
-
Molecular features of TNBC govern heterogeneity in the response to radiation and autophagy inhibition.Cell Death Dis. 2025 Jul 21;16(1):540. doi: 10.1038/s41419-025-07873-w. Cell Death Dis. 2025. PMID: 40691137 Free PMC article.
-
MUC1-C integrates activation of the IFN-γ pathway with suppression of the tumor immune microenvironment in triple-negative breast cancer.J Immunother Cancer. 2021 Jan;9(1):e002115. doi: 10.1136/jitc-2020-002115. J Immunother Cancer. 2021. PMID: 33495298 Free PMC article.
-
An update on cancer stem cell survival pathways involved in chemoresistance in triple-negative breast cancer.Future Oncol. 2025 Mar;21(6):715-735. doi: 10.1080/14796694.2025.2461443. Epub 2025 Feb 12. Future Oncol. 2025. PMID: 39936282 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Onkar SS, Carleton NM, Lucas PC, Bruno TC, Lee AV, Vignali DAA, et al. The great immune escape: understanding the divergent immune response in breast cancer subtypes. Cancer Discov. (2023) 13:23–40. doi: 10.1158/2159-8290.CD-22-0475, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

