The role of complement in normal pregnancy and preeclampsia
- PMID: 40777009
- PMCID: PMC12328418
- DOI: 10.3389/fimmu.2025.1643896
The role of complement in normal pregnancy and preeclampsia
Abstract
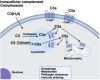
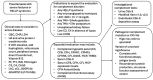
Preeclampsia affects 3-4% of pregnancies with adverse effects for both mother and child. Minimal therapeutic options are available, and biomarkers are urgently needed to identify those at greatest risk early in the pregnancy. Both the innate and adaptive immune systems are well regulated during normal pregnancy including the complement system. A functioning complement system with some degree of complement activation participates in proper placental development, ensuring a healthy pregnancy and assisting with host defense. However, aberrant complement activation can lead to adverse pregnancy outcomes such as preeclampsia. An overview of the complement system will be presented, along with review of the pre-clinical literature in animal models providing evidence for complement involvement in maintaining a normal pregnancy and contributing to symptoms of preeclampsia. In addition, clinical studies with evaluation of complement biomarkers in plasma and urine implicate complement dysregulation in the pathophysiology of subtypes of preeclampsia including HELLP (hemolysis, elevated liver enzymes and low platelet count) syndrome. Recent studies on the genetics of complement dysregulation in preeclampsia will be reviewed, along with updates on use of recently developed complement therapeutics. The potential utility of evaluating complement activation or manipulating complement during pregnancy will be discussed in view of the successful use of complement therapeutics in pregnancy in other immune diseases.
Keywords: complement; fetal development; innate immunity; preeclampsia; pregnancy; pregnancy loss.
Copyright © 2025 Burwick, Java and Regal.
Conflict of interest statement
AJ: Medical advisory board for Alexion pharmaceuticals and Novartis pharmaceuticals. Serves as PI for Novartis. Consultant for Apellis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Escudero-Esparza A, Kalchishkova N, Kurbasic E, Jiang WG, Blom AM. The novel complement inhibitor human CUB and Sushi multiple domains 1 (CSMD1) protein promotes factor I-mediated degradation of C4b and C3b and inhibits the membrane attack complex assembly. FASEB J. (2013) 27:5083–93. doi: 10.1096/fj.13-230706, PMID: - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

