Emerging IO checkpoints in gastrointestinal oncology
- PMID: 40777027
- PMCID: PMC12328307
- DOI: 10.3389/fimmu.2025.1575713
Emerging IO checkpoints in gastrointestinal oncology
Abstract
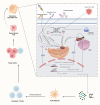
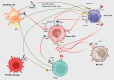
Recent progress in immunotherapy has significantly altered the therapeutic approach for gastrointestinal cancers, which are historically challenging due to their intricate pathologies and unfavorable outcomes. This review emphasizes the growing importance of immune checkpoints like TIGIT, VISTA, GITR, STING, and TIM-3 in the treatment of gastrointestinal oncology. These checkpoints are crucial elements within the tumor microenvironment, presenting new therapeutic possibilities. Studies show that TIGIT and GITR regulate the functions of T cells and NK cells, while the VISTA and STING pathways boost the body's anti-tumor responses. TIM-3 is linked with T cell fatigue, highlighting its potential as a target to counteract immune evasion mechanisms. Integrating these immune checkpoints with traditional treatments could result in more customized and effective therapeutic approaches. This detailed review seeks to explore the changing field of immune checkpoint research, offering insights from molecular biology to clinical practice, and envisioning a future where advanced treatment methods greatly enhance patient outcomes in GI cancers.
Keywords: GITR; STING; TIGIT; TIM-3; VISTA; cancer immunotherapy; gastrointestinal oncology; immune checkpoints.
Copyright © 2025 Tojjari, Saeed and Cavalcante.
Conflict of interest statement
AS reports research grants to institution from AstraZeneca, Bristol Myers Squibb, Merck, Clovis, Exelixis, Actuate Therapeutics, Incyte Corporation, Daiichi Sankyo, Five Prime Therapeutics, Amgen, Innovent Biologics, Dragonfly Therapeutics, KAHR Medical, BioNTech, and advisory board fees from Merck, AstraZeneca, Bristol Myers Squibb, Exelixis, Taiho, and Pfizer; Ludimila Cavalcante Consulting or Advisory Role: Pliant Therapeutics, Janssen, and CDR-Life. Stock and Other Ownership Interests: Actuate Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
New horizons in B-cell lymphoma immunotherapy: From immune checkpoints to precision medicine.Neoplasia. 2025 Sep;67:101206. doi: 10.1016/j.neo.2025.101206. Epub 2025 Jul 1. Neoplasia. 2025. PMID: 40592237 Free PMC article. Review.
-
TIGIT, as a potential immune checkpoint target for immunotherapy of breast cancer.Med Oncol. 2025 Aug 5;42(9):407. doi: 10.1007/s12032-025-02955-3. Med Oncol. 2025. PMID: 40762903 Review.
-
GITR and TIGIT immunotherapy provokes divergent multicellular responses in the tumor microenvironment of gastrointestinal cancers.Genome Med. 2023 Nov 26;15(1):100. doi: 10.1186/s13073-023-01259-3. Genome Med. 2023. PMID: 38008725 Free PMC article.
-
Interplay between tumor mutation burden and the tumor microenvironment predicts the prognosis of pan-cancer anti-PD-1/PD-L1 therapy.Front Immunol. 2025 Jul 24;16:1557461. doi: 10.3389/fimmu.2025.1557461. eCollection 2025. Front Immunol. 2025. PMID: 40777041 Free PMC article.
-
The AML immune paradox: decoding escape pathways and pioneering checkpoint, vaccine, and combination strategies.Clin Exp Med. 2025 Jul 9;25(1):240. doi: 10.1007/s10238-025-01795-9. Clin Exp Med. 2025. PMID: 40634759 Free PMC article. Review.
References
-
- Novartis . Novartis receives FDA fast track designation for sabatolimab (MBG453) in myelodysplastic syndromes. Novartis News Releases. (2021).
-
- Desai A, Subbiah V, Dimou A, Deshane J, Goliwas K, Ponnazhagan S, et al. Exploring the potential of combination immune checkpoint strategies in non-small cell lung cancer (NSCLC). J Clin Oncol. (2023) 41(16_suppl):9037. doi: 10.1200/JCO.2023.41.16_suppl.9037 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

