Response surface optimization for cadmium biosorption onto the pre-treated biomass of red algae Digenia simplex as a sustainable indigenous biosorbent
- PMID: 40777079
- PMCID: PMC12330821
- DOI: 10.7717/peerj.19776
Response surface optimization for cadmium biosorption onto the pre-treated biomass of red algae Digenia simplex as a sustainable indigenous biosorbent
Abstract
Background: Cadmium pollution from industrial effluent can cause major health concerns, so it must be removed from wastewater prior to disposal. The objective of this study was to remove cadmium (Cd2+) from aquatic environments using red macroalgae Digenia simplex pretreated with calcium chloride (CaCl2) (DSC).
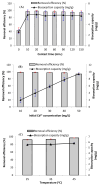
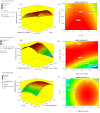
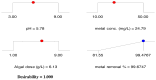
Methods: Batch adsorption studies were carried out to evaluate the individual impacts of adsorbent-metal contact time, cadmium concentration, and temperature on the cadmium removal efficiency and biosorption capacity. The Box-Benhken experimental design of response surface methodology was also used to investigate the relationship between different factors (pH, Cd2+ concentration and algal dose) and the cadmium removal efficiency of pretreated D. simplex.
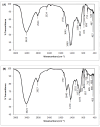
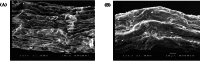
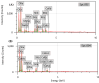
Results: The highest removal efficiency of 97.27% was achieved by combining different optimal parameters, including pH 5.78, initial Cd2+ concentration of 24.79 mg/L, and adsorbent dosage of 6.13 g/L. Moreover, cadmium removal from agricultural wastewater samples by pretreated D. simplex was evaluated under the optimal conditions, and the removal rate excessed 97%. Kinetic and isotherm investigations showed that the pseudo-second-order, Freundlich, Langmuir, and Dubinin-Radushkevich models of cadmium biosorption on pretreated algal biomass correlated well with the experimental biosorption data, implying that the biosorption of Cd2+ is a homogeneous monolayer and multilayer chemisorption process. The equilibrium isotherm data indicated that the biosorption capacity of the biosorbent was 11.16 mg/g as determined by the Langmuir model. Furthermore, the biosorption process was evaluated as an endothermic process with entropy and enthalpy values of 0.134 kJ/mol K and 38.01 kJ/mol, respectively. The functional groups, surface morphology, and elemental composition of the algal biomass were investigated, revealing the porous nature of the cell surface and the abundance of functional groups responsible for the Cd2+ biosorption process. These results suggest that DSC biomass can be used as a biosorbent for the effective removal of Cd2+ ions from effluent due to its availability and strong biosorption capability.
Keywords: Biosorption; Cadmium ion; Calcium chloride; Digenia simplex; Optimization; Response surface methodology.
©2025 Hassan et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures

References
-
- Abbou B, Lebkiri İ, Ouaddarı H, Elkhattabi O, Habsaouı A, Lebkırı A. Kinetic and thermodynamic study on adsorption of cadmium from aqueous solutions using natural clay. Journal of the Turkish Chemical Society, Section A: Chemistry. 2021;8(2):677–692. doi: 10.18596/jotcsa.882016. - DOI
-
- Al-Zaban MI, Alharbi NK, Albarakaty FM, Alharthi S, Hassan SHA, Fawzy MA. Experimental modeling investigations on the biosorption of methyl violet 2B dye by the brown seaweed Cystoseira tamariscifolia. Sustainability. 2022;14(9):5285. doi: 10.3390/su14095285. - DOI
-
- Aloufi AS, Al Riyami B, Fawzy MA, Al-Yasi HM, Koutb M, Hassan SH. Model-assisted optimization of cobalt biosorption on macroalgae Padina pavonica for wastewater treatment. Water. 2024;16(6):887. doi: 10.3390/w16060887. - DOI
-
- Alsawalha M. An approach utilizing the Response Surface Methodology (RSM) to optimize adsorption-desorption of natural saudi arabian diatomite-with the box–behnken design technique. Arabian Journal of Chemistry. 2023;16(1):104413. doi: 10.1016/j.arabjc.2022.104413. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

