This is a preprint.
Comparative Proteomic Profiling of Receptor Kinase Signaling Reveals Key Trafficking Components Enforcing Plant Stomatal Development
- PMID: 40777324
- PMCID: PMC12330694
- DOI: 10.1101/2025.07.20.665823
Comparative Proteomic Profiling of Receptor Kinase Signaling Reveals Key Trafficking Components Enforcing Plant Stomatal Development
Abstract
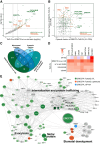
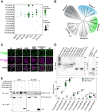
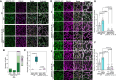
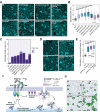
Receptor kinases are pivotal for growth, development, and environmental response of plants. Yet, their regulatory mechanisms and spatial dynamics are still underexplored. The ERECTA-family receptor kinases coordinate diverse developmental processes, including stomatal development. To understand the proteomic landscape of the ERECTA-mediated signaling pathways, we here report comparative analyses of the ERECTA interactome and proximitome by epitope-tagged affinity-purification (ET-AP) and TurboID-based proximity labeling (TbID-PL) mass-spectrometry, respectively. While ET-AP successfully recovered receptor complex components (e.g., TOO MANY MOUTHS), TbID-PL effectively captured transient associations with the components of endosomal trafficking, i.e. clathrin-mediated endocytosis (CME) machinery. We further identify that specific subfamily members of phosphatidylinositol-binding clathrin assembly proteins (PICALMs) interact with and synergistically regulate ERECTA internalization. Mutations of these PICALMs impair ERECTA endocytosis and lead to excessive stomatal clustering. Taken together, our work provides a proteomic atlas of the ERECTA signaling network and suggests that timely removal of receptor kinase by the endocytosis machinery is essential for active signal transduction enforcing stomatal patterning.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures


Similar articles
-
Comprehensive identification of cotton EPF/EPFL receptors and functional characterization of the GhEPFL1-1-GhER1 module in drought tolerance.BMC Plant Biol. 2025 Jul 11;25(1):901. doi: 10.1186/s12870-025-06797-z. BMC Plant Biol. 2025. PMID: 40646466 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Rabies virus utilizes neuropilin 2 as an endocytic receptor to trigger TGFBR1-mediated actin polymerization.J Virol. 2025 Jul 22;99(7):e0063825. doi: 10.1128/jvi.00638-25. Epub 2025 Jun 25. J Virol. 2025. PMID: 40558096 Free PMC article.
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
-
From gene to mechanics: a comprehensive insight into the mechanobiology of LMNA mutations in cardiomyopathy.Cell Commun Signal. 2024 Mar 27;22(1):197. doi: 10.1186/s12964-024-01546-5. Cell Commun Signal. 2024. PMID: 38539233 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
