This is a preprint.
A Microphysiologic Model of the Cervical Epithelium Recapitulates Microbial, Immunologic, and Pathogenic Properties of Sexually Transmitted Infections
- PMID: 40777327
- PMCID: PMC12330506
- DOI: 10.1101/2025.07.21.665989
A Microphysiologic Model of the Cervical Epithelium Recapitulates Microbial, Immunologic, and Pathogenic Properties of Sexually Transmitted Infections
Abstract
Sexually transmitted infections (STIs) of the cervicovaginal mucosa are among the most common global infections. Clinical studies have revealed that susceptibility to STIs and the subsequent host responses they elicit are frequently associated with vaginal microbiota compositions that facilitate infection. Current monolayer cell culture and animal models fail to reproduce the multilevel complexity required to investigate these relationships simultaneously and/or with sufficient physiological relevance. To address this limitation, we have developed a microphysiologic system (MPS) that models human cervical tissue, its microbiota, and is susceptible to infection by two prominent genital pathogens, Chlamydia trachomatis and Neisseria gonorrhoeae. Significantly, this MPS platform recapitulates essential dynamic, polymicrobial, immune, and pathogenic features of chlamydial and gonococcal infections as they occur in humans. The low-cost MPS device requires no specialized equipment or specific expertise and was experimentally validated for both chlamydial and gonococcal infections across multiple non-engineering, remotely located laboratories, demonstrating its transferability and reproducibility. The MPS platform described herein provides a novel tool for expanded research into genital infections in a reconstituted system that closely mimics the cervical epithelium, a significant advance over existing models.
Keywords: Microphysiologic model; cervix; chlamydia; gonorrhea; host-pathogen interactions; microbiome; organ on a chip.
Figures
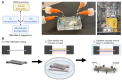
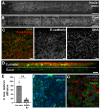

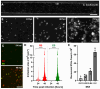
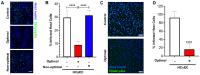
References
-
- CDC. Sexually Transmitted Infections Prevalence, Incidence, and Cost Estimates in the United States, April 2024.
-
- Chesson Harrell W., Mayaud Philippe, and Aral Sevgi O.. Sexually Transmitted Infections: Impact and Cost-Effectiveness of Prevention. In Holmes King K., Bertozzi Stefano, Bloom Barry R., and Prabhat Jha, editors, Major Infectious Diseases. The International Bank for Reconstruction and Development / The World Bank, Washington (DC), 3rd edition, 2017. ISBN 978-1-4648-0524-0978-1-4648-0525-7. - PubMed
-
- Global and regional STI estimates.
-
- Wiesenfeld Harold C., Hillier Sharon L., Krohn Marijane A., Landers Daniel V., and Sweet Richard L.. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 36(5):663–668, March 2003. ISSN 1537-6591. doi: 10.1086/367658. - DOI - PubMed
-
- Tamarelle J., Thiébaut A. C. M., de Barbeyrac B., Bébéar C., Ravel J., and Delarocque-Astagneau E.. The vaginal microbiota and its association with human papillomavirus, Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium infections: a systematic review and meta-analysis. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases, 25(1):35–47, January 2019. ISSN 1469-0691. doi: 10.1016/j.cmi.2018.04.019. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
