This is a preprint.
Response of UBR-box E3 ubiquitin ligases and protein quality control pathways to perturbations in protein synthesis and skeletal muscle size
- PMID: 40777329
- PMCID: PMC12330723
- DOI: 10.1101/2025.07.23.666188
Response of UBR-box E3 ubiquitin ligases and protein quality control pathways to perturbations in protein synthesis and skeletal muscle size
Abstract
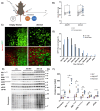
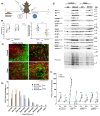
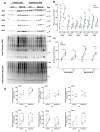
The N-degron pathway contributes to proteolysis by targeting N-terminal residues of destabilized proteins via E3 ligases that contain a UBR-box domain. Emerging evidence suggests the UBR-box family of E3 ubiquitin ligases (UBR1-7) are involved in the positive regulation of skeletal muscle mass. The purpose of this study was to explore the role of UBR-box E3 ubiquitin ligases under enhanced protein synthesis and skeletal muscle growth conditions. Cohorts of adult male mice were electroporated with constitutively active Akt (Akt-CA) or UBR5 RNAi constructs with a rapamycin diet intervention for 7 and 30 days, respectively. In addition, the UBR-box family was studied during the regrowth phase post nerve crush induced inactivity. Skeletal muscle growth with Akt-CA or regrowth following inactivity increased protein abundance of UBR1, UBR2, UBR4, UBR5 and UBR7. This occurred with corresponding increases in Akt-mTORC1/S6K and MAPK/p90RSK signaling and protein synthesis. The increases in UBR-box E3s, ubiquitination, and proteasomal activity occurred independently of mTORC1 activity and were associated with increases in markers related to autophagy, ER-stress, and protein quality control pathways. Finally, while UBR5 knockdown (KD) evokes atrophy, it occurs together with hyperactivation of mTORC1 and protein synthesis. In UBR5 KD muscles, we identified an increase in protein abundance for UBR2, UBR4 and UBR7, which may highlight a compensatory response to maintain proteome integrity. Future studies will seek to understand the role of UBR-box E3s towards protein quality control in skeletal muscle plasticity.
Keywords: N-degron pathway; UBR5; hypertrophy; proteasome system.
Conflict of interest statement
Conflict of Interest SCB is on the scientific advisory board for Emmyon Inc. All other authors declare that they do not have a conflict of interest.
Figures



Similar articles
-
An alternative pocket for binding the N-degrons by the UBR1 and UBR2 ubiquitin E3 ligases.Protein Sci. 2025 Aug;34(8):e70248. doi: 10.1002/pro.70248. Protein Sci. 2025. PMID: 40880185 Free PMC article.
-
A family of mammalian E3 ubiquitin ligases that contain the UBR box motif and recognize N-degrons.Mol Cell Biol. 2005 Aug;25(16):7120-36. doi: 10.1128/MCB.25.16.7120-7136.2005. Mol Cell Biol. 2005. PMID: 16055722 Free PMC article.
-
Heterozygous UBR5 variants result in a neurodevelopmental syndrome with developmental delay, autism, and intellectual disability.Am J Hum Genet. 2025 Jan 2;112(1):75-86. doi: 10.1016/j.ajhg.2024.11.009. Epub 2024 Dec 24. Am J Hum Genet. 2025. PMID: 39721588 Free PMC article.
-
Signaling Pathways Regulated by UBR Box-Containing E3 Ligases.Int J Mol Sci. 2021 Aug 3;22(15):8323. doi: 10.3390/ijms22158323. Int J Mol Sci. 2021. PMID: 34361089 Free PMC article. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Baehr LM, Hughes DC, Waddell DS, and Bodine SC. Snapshot: Skeletal muscle atrophy. Cell 185: 1618–1618. e1611, 2022. - PubMed
-
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, and Glass DJ. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature cell biology 3: 1014–1019, 2001. - PubMed
-
- Baar K, and Esser K. Phosphorylation of p70S6kcorrelates with increased skeletal muscle mass following resistance exercise. American Journal of Physiology-Cell Physiology 276: C120–C127, 1999. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
