This is a preprint.
Fusiform Cells in the Dorsal Cochlear Nucleus Change Intrinsic Electrophysiological Properties and Morphologically Remodel Their Basal Dendrites with Age
- PMID: 40777380
- PMCID: PMC12330737
- DOI: 10.1101/2025.07.16.665173
Fusiform Cells in the Dorsal Cochlear Nucleus Change Intrinsic Electrophysiological Properties and Morphologically Remodel Their Basal Dendrites with Age
Abstract
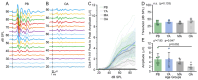
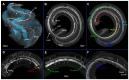
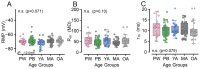
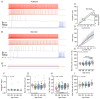
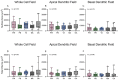
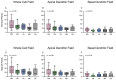
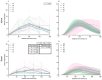
Age-related hearing loss (ARHL) is the most common cause of sensorineural hearing loss. The cochlear nucleus, the first central auditory structure to receive input from the cochlea, has been shown to be disrupted by ARHL. Fusiform cells (FC), the principal output cell of the dorsal part of the cochlear nucleus (DCN), mature physiologically during hearing onset. Specifically, FCs increase in rate of action potential (AP) rise and decay, stabilizing by postnatal day 14 (P14) in mice. However, whether FC intrinsic electrophysiological properties and morphological characteristics continue to change throughout the life of mice, and how they change due to ARHL, is unknown. We characterized electrophysiological and morphological properties of FCs from CBA/CaJ mice at five stages of age: preweaning (P15-20), pubescent (P21-49), young adult (P50-179), mature adult (P180-364), and old adult (P550-578). Our old adult mice had smaller auditory brainstem evoked response amplitudes and loss of some hair cells, indicative of ARHL onset. We observed no change in FC membrane properties with age. FCs from the old adult group had elevated firing rates, faster repolarization rates, and shorter AP half-widths. Morphologically, there was no change in FC soma shape or size. However, a significant decrease in basal dendritic arborization occurred between preweaning and pubescent ages, followed by an increase in our old adult group, suggesting age-dependent remodeling of the basal dendritic tree at the onset of ARHL. Together, these results suggest that FC physiology and morphology are relatively stable post weaning and become altered during the onset of ARHL.
Keywords: Cochlear Nucleus; Pyramidal Cell; hearing loss; intrinsic excitability; neuron morphology.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
