This is a preprint.
Functional Connectome of Superagers Reveals Early Markers of Resilience and Vulnerability to Alzheimer's Disease
- PMID: 40777400
- PMCID: PMC12330726
- DOI: 10.1101/2025.07.20.665707
Functional Connectome of Superagers Reveals Early Markers of Resilience and Vulnerability to Alzheimer's Disease
Abstract
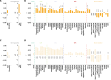
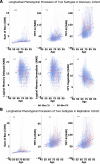
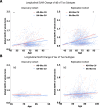
As populations age, identifying the neurobiological basis of cognitive resilience is critical for delaying or preventing Alzheimer's disease (AD). While most older adults experience memory decline, a subset known as superagers (SA) maintains youthful memory into late life, offering a unique window into protective mechanisms against neurodegeneration. Here, we identified a functional connectivity (FC) signature, termed Alzheimer's-resilient connectome (ARC), that robustly differentiates SA from age-matched patients with AD. Using resting-state fMRI in a discovery cohort (N = 290), we identified ARC derived from machine learning classifiers that distinguished SA from AD with high accuracy (AUC = 0.85), and validated the replicability of the ARC in an independent replication cohort (N = 143). ARC involved prefrontal, temporal and insular networks and was strongly associated with brain age. When applied to cognitively unimpaired (CU) adults (discovery cohort: N = 818 and replication cohort: N = 497), ARC-based subtyping revealed SA-like and AD-like subgroups with similar baseline cognitive performance but markedly divergent longitudinal trajectories. SA-like CU individuals showed slower cognitive decline, reduced amyloid-β accumulation, and lower risk of conversion to mild cognitive impairment and AD, reinforcing the ARC signature as a potential early indicator of resilience. Genome-wide association analysis identified CLYBL and FRMD6 as novel genetic modulators associated with these divergent aging phenotypes. Together, our findings position ARC as a sensitive and generalizable biomarker of resilience, enabling early risk stratification and precision prevention for AD.
Conflict of interest statement
Competing Interests G.A.F. received monetary compensation for consulting work for SynapseBio AI and owns equity in Alto Neuroscience. None of the other authors declare any competing interests.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
