This is a preprint.
BIOCOMPATIBILITY OF LARGE-AREA 2-DIMENSIONAL ELECTRONIC MATERIALS WITH NEURAL STEM CELLS
- PMID: 40777496
- PMCID: PMC12330722
- DOI: 10.1101/2025.07.19.665698
BIOCOMPATIBILITY OF LARGE-AREA 2-DIMENSIONAL ELECTRONIC MATERIALS WITH NEURAL STEM CELLS
Abstract
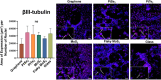
Two-dimensional (2D) electronic materials hold immense promise for next-generation bio/neuro-electronic interfaces, but their biocompatibility has remained uncertain due to conflicting reports from studies focused on exfoliated flakes and suspensions. In this work, we present a comprehensive in vitro evaluation of electronic-grade large-area, chemical vapor deposition (CVD)-grown 2D materials - including platinum diselenide (PtSe2), platinum ditelluride (PtTe2), molybdenum disulfide (MoS2), and graphene - as substrates for mouse neural stem cell culture. Across all CVD-grown materials, the stem cells exhibited outstanding viability, with no significant differences in metabolic activity or live/apoptotic cell ratios compared to laminin-coated glass controls (p > 0.05). Importantly, these large-area 2D materials robustly supported neuronal differentiation, as evidenced by widespread βIII-tubulin expression. Strikingly, we found that flaky MoS2 promoted significantly greater neuronal maturation (>75% NeuN+ neurons) than any other substrate tested (25-50% NeuN+; p < 0.05), revealing the critical influence of material format on bioactivity. While PtSe2 showed a tendency to promote glial lineage differentiation, our findings firmly establish large-area CVD-grown 2D materials as biocompatible, tunable platforms for neural interfacing, paving the way for their integration into advanced bio/neuro-electronic devices.
Keywords: ApoLive-Glo Assay; Cell-viability; Graphene; Immunocytochemistry (ICC); Live-Dead Imaging; MoS2; PtSe2; PtTe2.
Figures




References
-
- Kovach Kyle M., et al. “High-throughput in vitro assay to evaluate the cytotoxicity of liberated platinum compounds for stimulating neural electrodes.” Journal of neuroscience methods 273 (2016). - PubMed
-
- Lee Uhn, et al. “Cytotoxicity of gold nanoparticles in human neural precursor cells and rat cerebral cortex.” Journal of bioscience and bioengineering 121.3 (2016). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
