Follow-up study of vonoprazan maintenance therapy for reflux esophagitis: A 96-week evaluation in patients with PPI-refractory disease
- PMID: 40777627
- PMCID: PMC12329401
- DOI: 10.3892/br.2025.2038
Follow-up study of vonoprazan maintenance therapy for reflux esophagitis: A 96-week evaluation in patients with PPI-refractory disease
Abstract
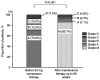
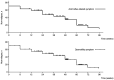
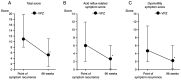
Vonoprazan is a potassium-competitive acid blocker that provides more potent and sustained acid suppression than conventional proton pump inhibitors (PPIs). Vonoprazan may serve a role in the long-term management of gastroesophageal reflux disease (GERD), particularly in patients with reflux esophagitis (RE) who are unresponsive to PPIs. The present study aimed to evaluate the safety, tolerability and efficacy of vonoprazan over a 96-week period in patients with PPI-refractory RE. Initially, 74 patients received vonoprazan 20 mg once daily for 4 weeks. Patients who demonstrated mucosal healing transitioned to vonoprazan 10 mg once daily for a 48-week maintenance phase. Of these, 43 patients continued therapy for an additional 48 weeks. Endoscopic evaluation, symptom scores using the Frequency Scale for the Symptoms of GERD and serum gastrin levels were monitored to assess treatment outcomes and safety. By the end of the 96-week maintenance period, 85.7% of patients who completed follow-up showed no recurrence of mucosal lesions. Among those who discontinued therapy following symptom resolution, 45.8% experienced symptom relapse; however, these patients responded well to reintroduction of vonoprazan. Although serum gastrin levels in the continuous maintenance therapy remained elevated, no adverse events such as carcinoid tumors were reported. These findings suggested that vonoprazan was both effective and well-tolerated as a long-term maintenance therapy for RE and may serve as a viable on-demand treatment strategy for relapse management. While the results are promising, they stem from a highly selected population. Therefore, further randomized, controlled trials are warranted to confirm the generalizability and long-term safety of vonoprazan in broader GERD populations.
Keywords: gastroesophageal reflux disease; maintenance treatment; recurrent reflux esophagitis; vonoprazan.
Copyright: © 2025 Mizuno et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Systematic review with network meta-analysis: indirect comparison of the efficacy of vonoprazan and proton-pump inhibitors for maintenance treatment of gastroesophageal reflux disease.J Gastroenterol. 2019 Aug;54(8):718-729. doi: 10.1007/s00535-019-01572-y. Epub 2019 Mar 27. J Gastroenterol. 2019. PMID: 30919071 Free PMC article.
-
Vonoprazan: A Review in Erosive Esophagitis and Non-Erosive Gastro-Esophageal Reflux Disease.Drugs. 2025 Jul;85(7):945-955. doi: 10.1007/s40265-025-02193-x. Epub 2025 May 19. Drugs. 2025. PMID: 40388079 Review.
-
Safety and efficacy of proton pump inhibitors in preterm infants with gastroesophageal reflux disease.Cochrane Database Syst Rev. 2025 Mar 11;3(3):CD015127. doi: 10.1002/14651858.CD015127.pub2. Cochrane Database Syst Rev. 2025. PMID: 40066936
-
Pharmacological treatment of children with gastro-oesophageal reflux.Cochrane Database Syst Rev. 2014 Nov 24;2014(11):CD008550. doi: 10.1002/14651858.CD008550.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2023 Aug 22;8:CD008550. doi: 10.1002/14651858.CD008550.pub3. PMID: 25419906 Free PMC article. Updated.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Alqassab DF, Hasan MJ, AlSaadoon AM, AlMuqahwi AJ, AlAwadhi FA, Bahram SA, Alsayyad AS. Prevalence and risk factors of gastroesophageal reflux disease among adults attending primary healthcare in Bahrain. J Family Med Prim Care. 2024;13:5758–5765. doi: 10.4103/jfmpc.jfmpc_968_24. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
