Aortitis after granulocyte-colony stimulating factor administration: A case report
- PMID: 40777628
- PMCID: PMC12329404
- DOI: 10.3892/br.2025.2039
Aortitis after granulocyte-colony stimulating factor administration: A case report
Abstract
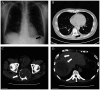
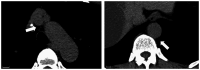
Granulocyte-colony stimulating factor (G-CSF) is commonly used to reduce neutropenia-related complications following chemotherapy. It is a glycoprotein that stimulates the production of granulocytes [white blood cells (WBCs) in the bone marrow. In the present study, the case of a 59-year-old man is presented who received chemotherapy (eribulin) for liver metastases from sacral chordoma and subsequently developed acute aortitis after the administration of G-CSF. Grade 3 neutropenia occurred on day eight of the fifth chemotherapy cycle, and pegfilgrastim was administered on day nine. A total of 3 days after pegfilgrastim administration, the patient developed a fever that persisted for 6 days. He visited our hospital on day 18 with abdominal pain and elevated WBC count and C-reactive protein levels. Febrile neutropenia was suspected, and antibiotics were administered. However, both blood and urinalysis cultures returned negative results, and antibiotics were ineffective. Contrast-enhanced computed tomography revealed a thickened wall of the brachiocephalic artery and abdominal aorta, consistent with aortitis. After discontinuing the antibiotics, the patient was monitored closely without further treatment. His condition improved within a few days; therefore, it was concluded that aortitis was induced by G-CSF.
Keywords: G-CSF; adverse event; aortitis; chemotherapy; chordoma.
Copyright © 2025, Spandidos Publications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Lyman GH, Dale DC, Culakova E, Poniewierski MS, Wolff DA, Kuderer NM, Huang M, Crawford J. The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: A systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2013;24:2475–2484. doi: 10.1093/annonc/mdt226. - DOI - PMC - PubMed
-
- National Comprehensive Cancer Network (NCCN): NCCN Clinical Practice Guidelines in Oncology: Hematopoietic Growth Factors. Version 1.2025. NCCN, Plymouth Meeting, PA, 2025. https://www.nccn.org. Accessed May 7, 2025.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
