Sex differences in drug-induced osteoporosis: a pharmacovigilance study based on the FAERS database
- PMID: 40777658
- PMCID: PMC12328446
- DOI: 10.3389/fpubh.2025.1630412
Sex differences in drug-induced osteoporosis: a pharmacovigilance study based on the FAERS database
Abstract
Background: Osteoporosis is a prevalent condition globally, often linked to a significant risk of fractures. Drug-induced osteoporosis (DIOP) is an increasingly recognized adverse effect of various medications, but the sex-specific risks and time-to-onset patterns remain inadequately understood. Addressing these gaps in knowledge is critical to improving patient safety and pharmacovigilance.
Objective: This study aimed to explore sex-related differences in DIOP, identify high-risk medications, and assess the onset patterns of osteoporosis-related adverse events by analyzing data from the FDA Adverse Event Reporting System (FAERS) and validating the findings using the Canada Vigilance Adverse Reaction Online Database (Canada Vigilance ADR).
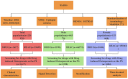
Methods: We analyzed adverse event reports from the FAERS database covering the period from Q1 2004 to Q4 2024. Drugs were standardized using the RxNorm drug terminology system, and adverse events were matched to MedDRA 27.1. Disproportionality analysis was conducted using Reporting Odds Ratio (ROR), Multi-item Gamma Poisson Shrinker (MGPS), and Bayesian Confidence Propagation Neural Network (BCPNN) methods. To validate our findings, we performed external validation using the Canada Vigilance ADR database. Stratified analyses by sex were performed to assess differences in drug-osteoporosis associations.
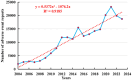
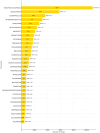
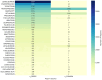
Results: A total of 236,928 osteoporosis-related reports were identified, with 64.6% of the reports coming from females. We identified 68 drugs associated with DIOP, including 15 male-specific and 26 female-specific potential risk drugs. Notable drugs such as tenofovir disoproxil and esomeprazole were linked to both sexes. Drugs like upadacitinib exhibited early-onset failure patterns, while others like tenofovir demonstrated cumulative risk patterns over prolonged use. External validation with the Canada Vigilance ADR confirmed 32 drugs with potential osteoporosis risks.
Conclusions: This study highlights important sex-specific differences in the risk of drug-induced osteoporosis and underscores the need for targeted pharmacovigilance strategies. The findings contribute to a more personalized approach to drug safety, promoting more informed decision-making regarding medication use in osteoporosis-prone populations.
Keywords: BCPNN; FAERS; drug-induced osteoporosis; mGPS; pharmacovigilance; sex-specific risk; time-to-onset analysis.
Copyright © 2025 Liu, Song, Liu, Song and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

