AAV6 vectors provide superior gene transfer compared to AAV9 vectors following intramyocardial administration
- PMID: 40777723
- PMCID: PMC12329528
- DOI: 10.1016/j.omtm.2025.101532
AAV6 vectors provide superior gene transfer compared to AAV9 vectors following intramyocardial administration
Abstract
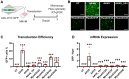
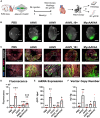
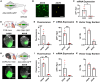
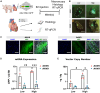
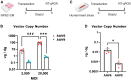
Cardiac gene therapy using adeno-associated viral (AAV) vectors holds great promise for treating heart diseases but would benefit from more potent AAV vectors. Vectors based on the AAV serotypes 6 and 9 have been used in pre-clinical gene therapy studies, yet the therapeutic outcomes varied depending on the experimental model and delivery route used. Here, we evaluated the transduction efficiency of AAV6, AAV9, and AAV9-derived MyoAAVs for local cardiac delivery. Vectors were tested in neonatal rat ventricular myocytes, and subsequently in mouse hearts by direct intramyocardial injection. Vector genome levels, mRNA expression levels, and fluorescence were measured. The AAV6 and AAV9 vectors were further validated in porcine hearts, human-induced pluripotent stem-cell-derived cardiomyocytes, and human atrial myocardial slices. In both rat cardiomyocytes and mouse hearts, AAV6 exhibited the highest transduction efficiency. Direct comparison of the AAV6 and AAV9 vectors in porcine and human models confirmed that AAV6 is more potent. In conclusion, AAV6 vectors are superior to AAV9 and its derivative vectors for cardiac transduction by direct intramyocardial injection. In addition, the in vivo transduction efficiency correlates with in vitro and ex vivo assays, thereby facilitating the development of more potent AAV variants for cardio-selective delivery methods.
Keywords: AAV vector; cardiac gene transfer; gene therapy; intramyocardial injection; transduction efficiency.
© 2025 The Author(s).
Conflict of interest statement
O.F.K., H.L.T., and G.J.J.B. are co-founders of PacingCure BV and report ownership interest in PacingCure BV. J.W. and E.E.B. are employees of PacingCure BV. T.J., V.M.C., and G.J.J.B. have pending patent applications related to this work.
Figures

References
-
- Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. - DOI - PMC - PubMed
-
- Konkalmatt P.R., Beyers R.J., O'Connor D.M., Xu Y., Seaman M.E., French B.A. Cardiac-selective expression of extracellular superoxide dismutase after systemic injection of adeno-associated virus 9 protects the heart against post-myocardial infarction left ventricular remodeling. Circ. Cardiovasc. Imaging. 2013;6:478–486. doi: 10.1161/CIRCIMAGING.112.000320. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

