Recent applications, future perspectives, and limitations of the CRISPR-Cas system
- PMID: 40777740
- PMCID: PMC12329533
- DOI: 10.1016/j.omtn.2025.102634
Recent applications, future perspectives, and limitations of the CRISPR-Cas system
Abstract
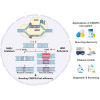
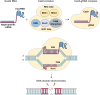
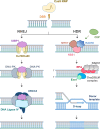
The CRISPR-Cas system has transformed our ability to edit and modify genomes in eukaryotic cells, offering unmatched precision and broad applicability. By utilizing a programmable RNA protein complex to introduce targeted double-strand breaks, the CRISPR-Cas system enables the correction of pathogenic mutations and the modulation of gene function with unprecedented efficiency. Its broad applicability spans the correction of inherited genetic defects through homology-directed repair to the disruption of deleterious alleles via non-homologous end joining. In this review, we first outline the molecular architecture and mechanistic basis of CRISPR-Cas9 and then consider its latest applications in modeling, drug screening, small-molecule-mediated editing, and treating hereditary, autoimmune, and oncological diseases. Emphasis is placed on the generation of disease-relevant cellular and animal models and on the potential of CRISPR-Cas9-mediated gene therapy to address hitherto intractable disorders. Finally, we discuss current challenges including off-target activity, gene editing efficiency, delivery constraints, and immunogenicity and highlight emerging strategies to overcome these hurdles and broaden the clinical impact of CRISPR-Cas systems.
Keywords: CRISPR-Cas9; DNA repair; MT: RNA/DNA Editing; cell engineering; drug screening; gene therapy; genetic editing efficiency.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Evolution of Prime Editing Systems: Move Forward to the Treatment of Hereditary Diseases.Curr Gene Ther. 2025;25(1):46-61. doi: 10.2174/0115665232295117240405070809. Curr Gene Ther. 2025. PMID: 38623982 Review.
-
Transferable approaches to CRISPR-Cas9 induced genome editing in non-model insects: a brief guide.Front Zool. 2025 Jul 7;22(1):13. doi: 10.1186/s12983-025-00566-2. Front Zool. 2025. PMID: 40624545 Free PMC article. Review.
-
Cell-Penetrating Peptides and CRISPR-Cas9: A Combined Strategy for Human Genetic Disease Therapy.Hum Gene Ther. 2024 Oct;35(19-20):781-797. doi: 10.1089/hum.2024.020. Hum Gene Ther. 2024. PMID: 39276086 Review.
-
CRISPR/Cas genome editing in soybean: challenges and new insights to overcome existing bottlenecks.J Adv Res. 2025 Jul;73:53-72. doi: 10.1016/j.jare.2024.08.024. Epub 2024 Aug 18. J Adv Res. 2025. PMID: 39163906 Free PMC article. Review.
-
Off-target interactions in the CRISPR-Cas9 Machinery: mechanisms and outcomes.Biochem Biophys Rep. 2025 Jul 5;43:102134. doi: 10.1016/j.bbrep.2025.102134. eCollection 2025 Sep. Biochem Biophys Rep. 2025. PMID: 40688512 Free PMC article. Review.
References
-
- Rath D., Amlinger L., Rath A., Lundgren M. The CRISPR-Cas immune system: biology, mechanisms and applications. Biochimie. 2015;117:119–128. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

