High false hepatitis C antibody positivity rate in a regionally-inclusive population of non-renumerated blood donors in Uganda
- PMID: 40777978
- PMCID: PMC12327096
- DOI: 10.4314/ahs.v24i3.6
High false hepatitis C antibody positivity rate in a regionally-inclusive population of non-renumerated blood donors in Uganda
Abstract
Background: Successful elimination of hepatitis as a public health threat by 2030 will partly rely on the availability and accessibility of affordable accurate disease testing platforms. In the past, testing of hepatitis C virus (HCV) in low resource settings of sub-Saharan Africa (SSA) has relied on anti-HCV testing using rapid diagnostic tests, chemiluminescent microparticle immunoassay (CMIA) and Enzyme-linked Immunosorbent Assays (ELISA) whose diagnostic accuracy has been sub-optimal. We determined the false positivity rate of a CMIA platform that is routinely used to screen donor blood for anti-HCV in Uganda.
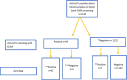
Methods: 1,216 CMIA-screened anti-HCV-positive blood donor samples at four regional Ugandan blood banks, were subjected to a third generation ELISA and subsequently to nucleic acid testing (NAT).
Results: Of the above 1,216 samples, 1,122 (92.2%) were negative on ELISA and thus deemed false positives. Active infection (NAT positive) was detected in 98 (8.0%). Presumed resolved infection was recorded among 3 (3.2%) of participants that remained positive on the ELISA platform but negative on NAT.
Conclusion: The Architect CMIA assay exhibited very low specificity for anti-HCV testing. In this context, this finding may suggest need to employ testing protocols that include NAT or a combination of tests with higher validity.
Keywords: High false hepatitis C; Uganda; antibody positivity rate; non-renumerated blood donors.
© 2024 Ochama P et al.
Figures
References
-
- Polaris Observatory, author. (http://cdsfound.org/polaris. )
-
- WHO Global Hepatitis Report. 2017. http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en.
-
- The Global Health Sector Strategy (GHSS) on viral hepatitis, author. https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
