Design and preparation of a new Ni/Arg@zeolite-Y nano-composite: investigation of its performance as a multi-functional and bio-organic catalyst for the one-pot synthesis of thieno[2,3- d]pyrimidinones
- PMID: 40778097
- PMCID: PMC12329655
- DOI: 10.1039/d5ra04062k
Design and preparation of a new Ni/Arg@zeolite-Y nano-composite: investigation of its performance as a multi-functional and bio-organic catalyst for the one-pot synthesis of thieno[2,3- d]pyrimidinones
Abstract
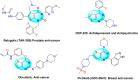
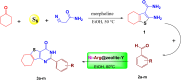
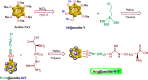
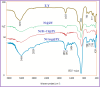
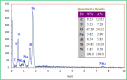
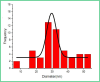
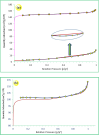
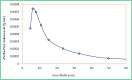
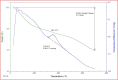
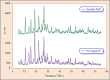
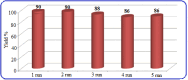
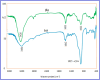
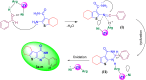
This paper describes the design and fabrication of a Ni/Arg@zeolite-Y nanocomposite, and investigates its application as a multifunctional nanocatalyst in the one-pot synthesis of thieno[2,3-d]pyrimidinone derivatives. Herein, nickel metal ions were stabilized on NaY zeolite through a sodium exchange process (Ni@zeolite-Y). Then, this catalyst was functionalized via the linker 3-chloropropyltriethoxysilane with the basic l-arginine amino acid (Arg@zeolite-NiY). The structure of the nano-catalyst was confirmed using techniques such as FT-IR, XRD, BET, FE-SEM, TGA-DTA, and EDX-MAP. The catalytic activity of this nanocomposite in the green synthesis of thieno-pyrimidines was evaluated. Initially, the feasibility of conducting the reaction with this nano-catalyst was assessed through a one-pot reaction involving the cyclization of 2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxamide with various aromatic aldehydes under different conditions. Subsequently, the reaction's generality for synthesizing thienopyrimidines under optimized conditions was successfully demonstrated. Key advantages of this project include the elimination of the toxic homogeneous catalyst hydrochloric acid and the use of green solvent ethanol. Additional benefits are the non-toxic nature, cost-effectiveness, recyclability of the nano-catalyst, ease of product isolation, high yield, and reduced reaction time.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no interest to influence the work reported in this paper.
Figures


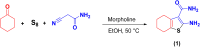
Similar articles
-
Periodic mesoporous organosilica functionalized with p-aminobenzenesulfonic acid: an efficient solid acid catalyst for one-pot synthesis of 5-substituted 1H-tetrazoles under eco-friendly conditions.Nanoscale Adv. 2025 May 19;7(15):4691-4709. doi: 10.1039/d5na00224a. eCollection 2025 Jul 22. Nanoscale Adv. 2025. PMID: 40575618 Free PMC article.
-
Natural asphalt oxide-grafted carboxylic acid: a sustainable heterogeneous catalyst for synthesis of pyrano[2,3-c]pyrazoles and 2-amino-3-cyanopyridines in water.RSC Adv. 2025 Jul 16;15(31):25274-25290. doi: 10.1039/d5ra03786g. eCollection 2025 Jul 15. RSC Adv. 2025. PMID: 40673260 Free PMC article.
-
Fabrication of Fe3O4@UiO-66-NH2-QCA-CuCl2 nanocomposites as a novel magnetic metal-organic framework catalyst for sustainable synthesis of 2,3-diarylquinolines.BMC Chem. 2025 Jul 3;19(1):189. doi: 10.1186/s13065-025-01516-z. BMC Chem. 2025. PMID: 40611331 Free PMC article.
-
Efficacy of nicergoline in dementia and other age associated forms of cognitive impairment.Cochrane Database Syst Rev. 2001;2001(4):CD003159. doi: 10.1002/14651858.CD003159. Cochrane Database Syst Rev. 2001. PMID: 11687175 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Solomon N. O. Simpa P. Adenekan O. A. Obasi S. C. Sustainable nanomaterials' role in green supply chains and environmental sustainability. Eng. Sci. Technol. J. 2024;5:1678–1694.
-
- Sher F. Ziani I. Hameed M. Ali S. Sulejmanović J. Advanced nanomaterials design and synthesis for accelerating sustainable biofuels production – A review. Curr. Opin. Green Sustainable Chem. 2024;47:100925.
-
- Yıldız Ü. Y., Keçili R. and Hussain C. M., Green and sustainable chemistry, Green Imprinted Materials, Elsevier, 2024, ch. 1, pp. 3–25
-
- Afolabi R. O. A comprehensive review of nanosystems' multifaceted applications in catalysis, energy, and the environment. J. Mol. Liq. 2024;397:124190.
-
- Ahmad I. Aftab M. A. Fatima A. Mekkey S. D. Melhi S. Ikram S. A comprehensive review on the advancement of transition metals incorporated on functional magnetic nanocomposites for the catalytic reduction and photocatalytic degradation of organic pollutants. Coord. Chem. Rev. 2024;514:215904.
LinkOut - more resources
Full Text Sources

